Microcarbons consist of small, simple carbon-containing molecules that form the building blocks for more complex organic compounds, while macrocarbons are large, complex molecules made up of many interconnected carbon atoms, often providing structural and functional roles in biological systems. Your understanding of their differences can help clarify how carbon's versatility supports both basic molecular functions and intricate biological architectures.
Table of Comparison
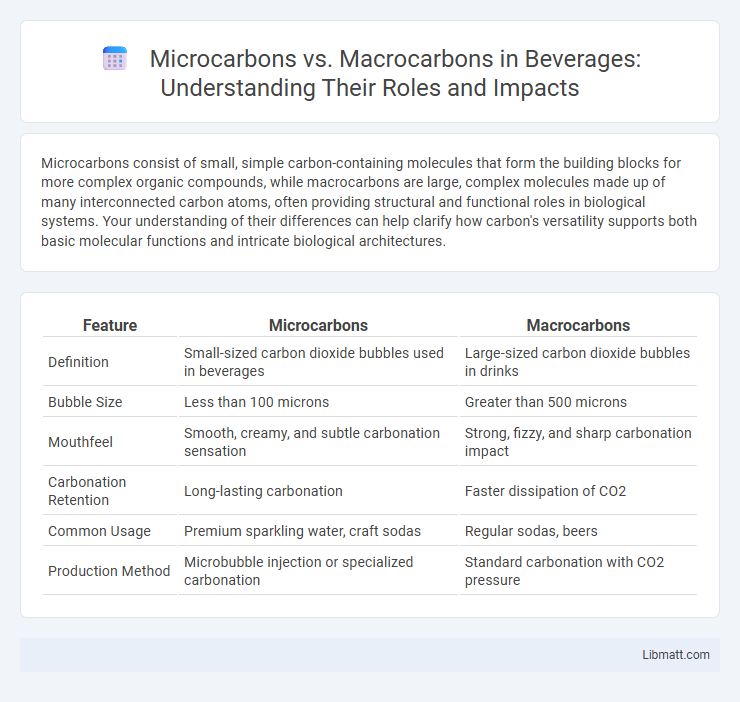
| Feature | Microcarbons | Macrocarbons |
|---|---|---|
| Definition | Small-sized carbon dioxide bubbles used in beverages | Large-sized carbon dioxide bubbles in drinks |
| Bubble Size | Less than 100 microns | Greater than 500 microns |
| Mouthfeel | Smooth, creamy, and subtle carbonation sensation | Strong, fizzy, and sharp carbonation impact |
| Carbonation Retention | Long-lasting carbonation | Faster dissipation of CO2 |
| Common Usage | Premium sparkling water, craft sodas | Regular sodas, beers |
| Production Method | Microbubble injection or specialized carbonation | Standard carbonation with CO2 pressure |
Introduction to Microcarbons and Macrocarbons
Microcarbons are tiny carbon-based molecules typically composed of fewer than 10 carbon atoms, playing crucial roles in chemical reactions and environmental processes. Macrocarbons, in contrast, consist of extensive carbon chains or rings, often forming polymers or complex organic compounds integral to materials science and biochemistry. Understanding the distinct properties of microcarbons and macrocarbons helps optimize your applications in pharmaceuticals, nanotechnology, and sustainable materials development.
Defining Microcarbons: Structure and Properties
Microcarbons are molecules composed of a small number of carbon atoms, typically fewer than 10, characterized by simple, well-defined structures such as methane or ethane. Their properties include lower molecular weight, higher volatility, and greater reactivity compared to macrocarbons, which consist of extensive carbon chains or networks like polymers or graphite. Understanding these structural differences helps you appreciate how microcarbons function in chemical reactions and industrial applications.
Macrocarbons Explained: Scale and Characteristics
Macrocarbons refer to large organic molecules primarily composed of carbon atoms arranged in extensive chains or rings, often found in materials like polymers and biomacromolecules. Their scale typically ranges from thousands to millions of atomic mass units, resulting in complex structural characteristics such as high molecular weight, diverse functional groups, and significant mechanical strength. Unlike microcarbons, which include small molecules or limited carbon structures, macrocarbons exhibit properties essential for applications in materials science, biology, and nanotechnology due to their size and structural complexity.
Key Differences Between Microcarbons and Macrocarbons
Microcarbons refer to carbon entities with microscopic dimensions, often found in nanotechnology and material sciences, while macrocarbons are large-scale carbon structures such as graphite and diamond. Microcarbons exhibit unique quantum properties and high surface area-to-volume ratios, impacting reactivity and strength, contrasting with the bulk physical properties of macrocarbons. Your choice between microcarbon and macrocarbon materials depends on the application requirements for conductivity, flexibility, or structural integrity.
Formation Processes: Microcarbons vs Macrocarbons
Microcarbons form through rapid, low-temperature pyrolysis or combustion of organic materials, resulting in small carbon clusters or soot particles. Macrocarbons develop via slower, high-temperature processes such as coalification or graphite formation, where extensive carbon atom bonding creates large, stable structures. The distinct thermal conditions and reaction times critically influence the size, morphology, and chemical properties of microcarbon and macrocarbon materials.
Applications in Industry and Technology
Microcarbons, due to their nanoscale size and high surface area, are essential in advanced electronics, energy storage, and catalysis, enabling enhanced conductivity and reactivity in devices such as supercapacitors and sensors. In contrast, macrocarbons, with their larger structural framework, find extensive applications in construction materials, carbon fibers for aerospace, and automotive industries where strength and durability are crucial. Both forms optimize material performance but cater to distinct technological demands based on their scale and physicochemical properties.
Environmental Impact of Microcarbons vs Macrocarbons
Microcarbons, such as microplastics and small particulate pollutants, have a pervasive environmental impact due to their ability to infiltrate ecosystems, harming aquatic life and entering the food chain. Macrocarbons, including larger plastic debris and fossil fuel-based carbon emissions, contribute significantly to habitat destruction and climate change through extensive pollution and greenhouse gas release. Understanding the differential impact between microcarbons and macrocarbons helps you prioritize mitigation strategies to reduce environmental contamination and protect biodiversity.
Analytical Techniques for Identification and Measurement
Microcarbons are typically analyzed using advanced spectroscopy methods such as Gas Chromatography-Mass Spectrometry (GC-MS) and Fourier Transform Infrared Spectroscopy (FTIR), which allow precise identification and quantification at trace levels. Macrocabons require techniques like Nuclear Magnetic Resonance (NMR) spectroscopy and High-Performance Liquid Chromatography (HPLC) for detailed structural analysis and concentration measurement. Both microcarbons and macrocabons benefit from complementary use of Thermal Desorption and Elemental Analysis to enhance accuracy and sensitivity in detection.
Future Trends and Innovations in Carbon Science
Future trends in carbon science emphasize the growing significance of microcarbons, such as graphene and carbon nanotubes, for their exceptional mechanical strength and electrical conductivity in advanced materials and electronics. Innovations focus on scalable synthesis methods and functionalization techniques to enhance microcarbon compatibility in energy storage, sensors, and biomedical applications. Meanwhile, macrocarbons like activated carbon continue evolving with improved porosity control and surface chemistry for environmental remediation and carbon capture technologies.
Conclusion: Choosing the Right Carbon Scale
Selecting the appropriate carbon scale depends on your project's scope and precision requirements. Microcarbons offer detailed insights at the molecular level, ideal for specialized scientific research or high-resolution analysis. Macrocarbons provide broader context for ecosystem-level studies or large-scale carbon cycling assessments, ensuring your data aligns with environmental goals and accuracy needs.
Microcarbons vs macrocarbons Infographic
 libmatt.com
libmatt.com