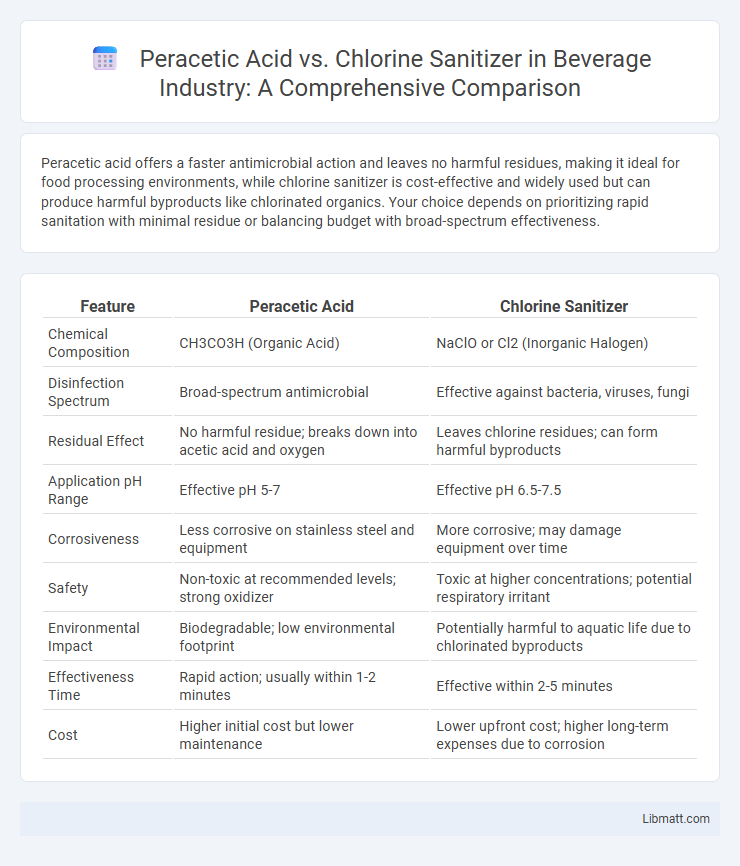
Peracetic acid offers a faster antimicrobial action and leaves no harmful residues, making it ideal for food processing environments, while chlorine sanitizer is cost-effective and widely used but can produce harmful byproducts like chlorinated organics. Your choice depends on prioritizing rapid sanitation with minimal residue or balancing budget with broad-spectrum effectiveness.
Table of Comparison
| Feature | Peracetic Acid | Chlorine Sanitizer |
|---|---|---|
| Chemical Composition | CH3CO3H (Organic Acid) | NaClO or Cl2 (Inorganic Halogen) |
| Disinfection Spectrum | Broad-spectrum antimicrobial | Effective against bacteria, viruses, fungi |
| Residual Effect | No harmful residue; breaks down into acetic acid and oxygen | Leaves chlorine residues; can form harmful byproducts |
| Application pH Range | Effective pH 5-7 | Effective pH 6.5-7.5 |
| Corrosiveness | Less corrosive on stainless steel and equipment | More corrosive; may damage equipment over time |
| Safety | Non-toxic at recommended levels; strong oxidizer | Toxic at higher concentrations; potential respiratory irritant |
| Environmental Impact | Biodegradable; low environmental footprint | Potentially harmful to aquatic life due to chlorinated byproducts |
| Effectiveness Time | Rapid action; usually within 1-2 minutes | Effective within 2-5 minutes |
| Cost | Higher initial cost but lower maintenance | Lower upfront cost; higher long-term expenses due to corrosion |
Introduction to Peracetic Acid and Chlorine Sanitizers
Peracetic acid and chlorine sanitizers are widely used disinfectants in food processing and healthcare industries due to their powerful antimicrobial properties. Peracetic acid, a mixture of acetic acid and hydrogen peroxide, offers rapid and effective pathogen reduction without leaving harmful residues, making it suitable for sensitive surfaces. Chlorine sanitizers, based on compounds like sodium hypochlorite, remain cost-effective and efficient for broad-spectrum disinfection but may cause corrosion and require cautious handling to maintain safety and efficacy.
Chemical Properties and Composition
Peracetic acid is a strong oxidizing agent composed of acetic acid and hydrogen peroxide, known for its rapid antimicrobial activity and environmentally friendly decomposition into non-toxic byproducts. Chlorine sanitizer primarily contains sodium hypochlorite or chlorine gas, releasing hypochlorous acid in water, which provides effective broad-spectrum disinfection but may form harmful chlorinated byproducts. The chemical stability of peracetic acid is lower than chlorine, requiring on-site generation, while chlorine sanitizers offer longer shelf life but pose higher risks of corrosion and toxic residue formation.
Mechanisms of Action
Peracetic acid acts as a powerful oxidizing agent, disrupting cell walls and denaturing proteins, leading to rapid microbial inactivation. Chlorine sanitizer functions by releasing hypochlorous acid, which penetrates microbial cell membranes and oxidizes critical enzymes and cellular components. Both disinfectants exhibit broad-spectrum antimicrobial activity but differ in their chemical interactions with pathogens and environmental stability.
Applications in Various Industries
Peracetic acid is widely used in the food and beverage, healthcare, and pharmaceutical industries due to its effectiveness in eliminating bacteria, viruses, and spores without leaving harmful residues. Chlorine sanitizers are commonly applied in water treatment, swimming pools, and wastewater management because of their cost-efficiency and strong oxidizing properties. Your choice between peracetic acid and chlorine sanitizers depends on the specific requirements of sanitation, such as contact time, material compatibility, and regulatory approvals.
Efficacy Against Microorganisms
Peracetic acid demonstrates superior efficacy against a broad spectrum of microorganisms, including bacteria, viruses, fungi, and spores, outperforming chlorine sanitizers in organic load conditions. Its rapid oxidation mechanism disrupts microbial cell walls more effectively than chlorine, leading to faster and more consistent microbial reduction. Studies show peracetic acid maintains potency at lower concentrations and shorter contact times, enhancing its reliability for sanitation in food processing and healthcare environments.
Safety and Handling Considerations
Peracetic acid offers a safer alternative to chlorine sanitizers due to its lower toxicity and reduced risk of harmful byproducts such as chlorinated organics. It requires careful handling with protective equipment as it is a strong oxidizer that can cause skin and respiratory irritation, but it decomposes into non-toxic acetic acid and water, minimizing environmental impact. Chlorine sanitizers, while effective, pose risks of hazardous chlorine gas release and corrosive effects, demanding strict ventilation and storage protocols to ensure user safety.
Environmental Impact and Biodegradability
Peracetic acid boasts a lower environmental impact compared to chlorine sanitizer due to its rapid biodegradability and decomposition into non-toxic byproducts like water, oxygen, and acetic acid. Chlorine sanitizer often forms harmful chlorinated compounds that persist in the environment and contribute to water pollution and toxicity in aquatic ecosystems. Choosing peracetic acid for sanitization supports a reduced ecological footprint and safer disposal practices for your facility.
Cost Comparison and Availability
Peracetic acid generally has a higher upfront cost compared to chlorine sanitizers, but it often reduces long-term expenses due to lower chemical usage and shorter contact times. Chlorine sanitizers are widely available and cost-effective, making them a common choice for many industries despite potential corrosion and odor issues. Availability for peracetic acid can be limited in some regions due to storage and handling regulations, while chlorine products are easily sourced globally.
Regulatory Standards and Approvals
Peracetic acid is approved by the U.S. Environmental Protection Agency (EPA) and meets NSF/ANSI Standard 60 for use in water treatment, emphasizing its strong oxidation properties and environmental safety. Chlorine sanitizers comply with EPA regulations and are widely accepted under FDA guidelines for food contact surfaces, but they often require careful management due to potential byproduct formation like chlorinated organics. Both sanitizers must adhere to strict regulatory standards involving concentration limits, contact time, and residue levels to ensure efficacy and safety in drinking water and food processing applications.
Choosing the Right Sanitizer for Your Needs
Peracetic acid offers rapid microbial kill with no harmful residues, making it ideal for food processing and healthcare where safety and environmental impact are priorities. Chlorine sanitizers are cost-effective and widely used for general surface disinfection but can produce harmful byproducts and lose efficacy in organic matter presence. Selecting between peracetic acid and chlorine depends on factors such as application environment, contact time, regulatory compliance, and the desired balance between effectiveness and sustainability.
Peracetic acid vs chlorine sanitizer Infographic
 libmatt.com
libmatt.com