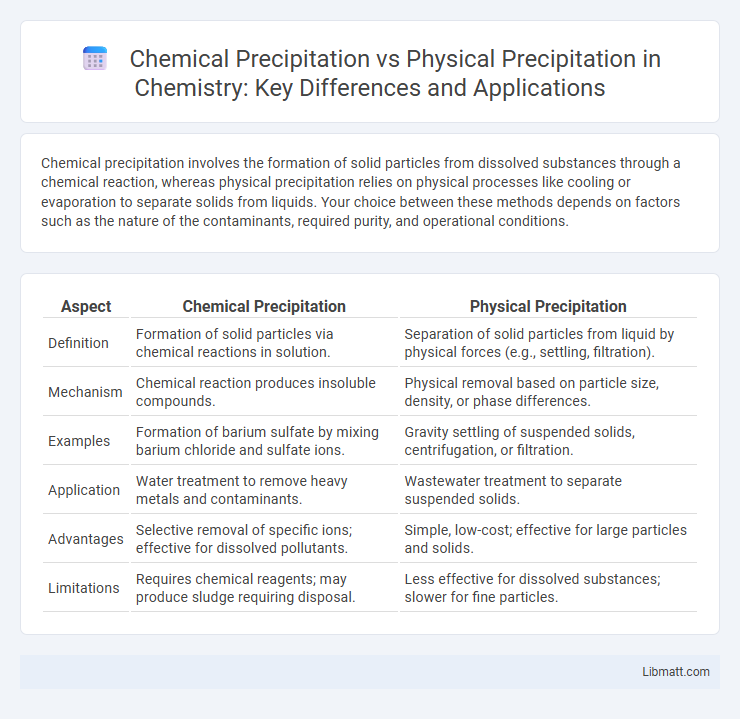
Chemical precipitation involves the formation of solid particles from dissolved substances through a chemical reaction, whereas physical precipitation relies on physical processes like cooling or evaporation to separate solids from liquids. Your choice between these methods depends on factors such as the nature of the contaminants, required purity, and operational conditions.
Table of Comparison
| Aspect | Chemical Precipitation | Physical Precipitation |
|---|---|---|
| Definition | Formation of solid particles via chemical reactions in solution. | Separation of solid particles from liquid by physical forces (e.g., settling, filtration). |
| Mechanism | Chemical reaction produces insoluble compounds. | Physical removal based on particle size, density, or phase differences. |
| Examples | Formation of barium sulfate by mixing barium chloride and sulfate ions. | Gravity settling of suspended solids, centrifugation, or filtration. |
| Application | Water treatment to remove heavy metals and contaminants. | Wastewater treatment to separate suspended solids. |
| Advantages | Selective removal of specific ions; effective for dissolved pollutants. | Simple, low-cost; effective for large particles and solids. |
| Limitations | Requires chemical reagents; may produce sludge requiring disposal. | Less effective for dissolved substances; slower for fine particles. |
Introduction to Chemical and Physical Precipitation
Chemical precipitation involves the transformation of dissolved substances into insoluble solids through chemical reactions, often by adding precipitating agents like lime or alum. Physical precipitation refers to the removal of suspended particles from water by processes such as sedimentation, where gravity allows heavier particles to settle out naturally. Understanding these distinctions helps optimize Your water treatment process by selecting the appropriate method based on the contaminants present.
Definitions: Chemical vs Physical Precipitation
Chemical precipitation involves the transformation of dissolved substances into solid particles through a chemical reaction, often resulting in the formation of insoluble compounds. Physical precipitation refers to the process where suspended particles aggregate and settle out of a liquid due to physical forces such as gravity or temperature changes, without any chemical alteration. Both methods are essential in water and wastewater treatment for removing contaminants, but chemical precipitation typically targets dissolved ions while physical precipitation deals with particulate matter.
Key Differences Between Chemical and Physical Precipitation
Chemical precipitation involves the transformation of dissolved substances into solid particles through chemical reactions, often used to remove contaminants from wastewater by forming insoluble compounds. Physical precipitation relies on physical processes such as sedimentation, filtration, or centrifugation to separate solid particles from liquids without altering their chemical composition. The key difference lies in chemical precipitation inducing a chemical change for contaminant removal, while physical precipitation depends solely on physical separation techniques.
Mechanisms of Chemical Precipitation
Chemical precipitation relies on chemical reactions that transform dissolved substances into solid particles, commonly through processes like hydrolysis, neutralization, or ion exchange. These reactions alter the solubility of specific ions, causing them to bond and form insoluble precipitates that can be separated from the solution. This mechanism contrasts with physical precipitation, which primarily depends on physical forces like gravity or filtration to remove suspended particles without altering their chemical nature.
Mechanisms of Physical Precipitation
Physical precipitation involves the process where particles or solutes separate from a solution due to changes in physical conditions such as temperature, pressure, or solubility without involving chemical reactions. Mechanisms include cooling, evaporation, or dissolving gases which reduce solubility, causing solids to aggregate and settle out. This method is often employed in water treatment to remove suspended solids and in industrial processes to recover valuable materials from solutions.
Common Applications of Chemical Precipitation
Chemical precipitation is widely used in wastewater treatment to remove heavy metals, phosphate, and other dissolved contaminants by forming insoluble compounds that can be filtered out. Industries such as mining, metal plating, and pharmaceuticals commonly apply chemical precipitation to manage and recover valuable metals, ensuring environmental compliance. This method is preferred over physical precipitation when precise removal of ionic pollutants or specific chemical species is required for effective purification.
Real-world Uses of Physical Precipitation
Physical precipitation methods such as sedimentation and centrifugation are widely used in water treatment plants to remove suspended solids and reduce turbidity efficiently. In industrial processes, physical precipitation techniques aid in separating particles from liquids without altering their chemical composition, commonly applied in wastewater treatment and mining operations. These methods provide cost-effective, scalable solutions for solid-liquid separation, enhancing process control and environmental compliance.
Advantages and Disadvantages of Chemical Precipitation
Chemical precipitation effectively removes dissolved metals and contaminants from wastewater by forming insoluble compounds, offering high removal efficiency and the ability to target specific pollutants. However, it generates chemical sludge that requires proper disposal and may involve higher operational costs due to reagent consumption. The method's dependence on precise pH control and chemical dosing can complicate process management compared to simpler physical precipitation techniques.
Pros and Cons of Physical Precipitation
Physical precipitation offers advantages such as simplicity, cost-effectiveness, and minimal chemical usage, making it suitable for large-scale water treatment applications. However, its limitations include lower efficiency in removing dissolved contaminants and potential reliance on precise temperature or pressure conditions. You should consider these trade-offs when selecting the appropriate precipitation method for your specific water purification needs.
Conclusion: Choosing Between Chemical and Physical Precipitation
Choosing between chemical and physical precipitation depends on factors such as contaminant type, treatment goals, and operational costs. Chemical precipitation effectively removes dissolved heavy metals and phosphorus through reagent addition, while physical precipitation primarily targets suspended solids via sedimentation or filtration. Optimal selection balances efficiency, cost, and system complexity to achieve desired water quality outcomes.
chemical precipitation vs physical precipitation Infographic
 libmatt.com
libmatt.com