Pervaporation offers selective separation of mixtures by membrane permeation and evaporation, efficiently removing volatile components without phase changes, while distillation relies on heating to separate substances based on boiling point differences. Your choice depends on factors like energy efficiency, separation selectivity, and the thermal sensitivity of the mixture components.
Table of Comparison
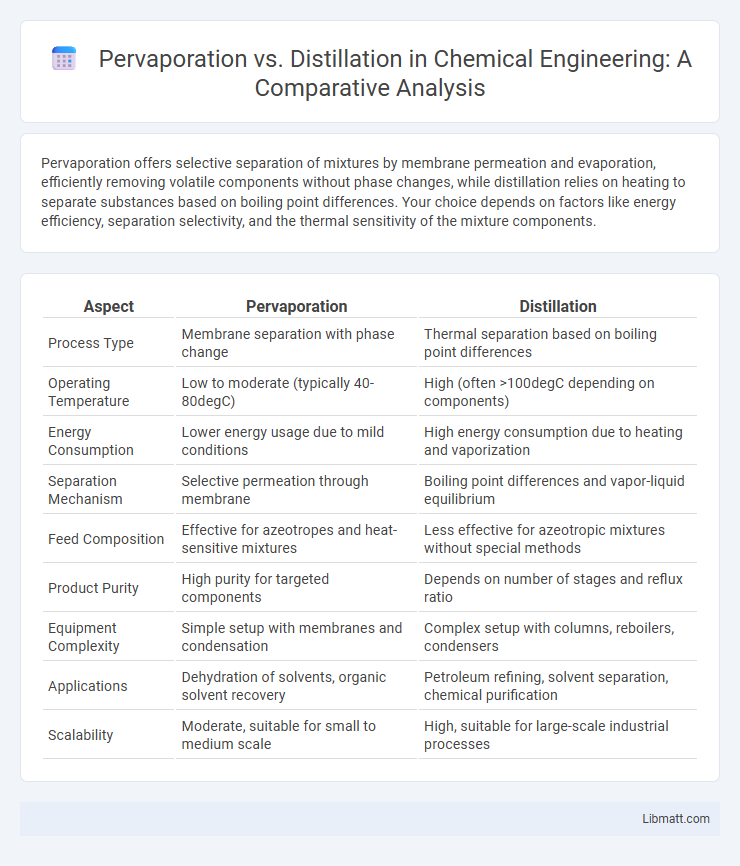
| Aspect | Pervaporation | Distillation |
|---|---|---|
| Process Type | Membrane separation with phase change | Thermal separation based on boiling point differences |
| Operating Temperature | Low to moderate (typically 40-80degC) | High (often >100degC depending on components) |
| Energy Consumption | Lower energy usage due to mild conditions | High energy consumption due to heating and vaporization |
| Separation Mechanism | Selective permeation through membrane | Boiling point differences and vapor-liquid equilibrium |
| Feed Composition | Effective for azeotropes and heat-sensitive mixtures | Less effective for azeotropic mixtures without special methods |
| Product Purity | High purity for targeted components | Depends on number of stages and reflux ratio |
| Equipment Complexity | Simple setup with membranes and condensation | Complex setup with columns, reboilers, condensers |
| Applications | Dehydration of solvents, organic solvent recovery | Petroleum refining, solvent separation, chemical purification |
| Scalability | Moderate, suitable for small to medium scale | High, suitable for large-scale industrial processes |
Introduction to Pervaporation and Distillation
Pervaporation is a membrane separation process that selectively removes certain components from liquid mixtures by partial vaporization through a permeable membrane, offering energy-efficient separation especially for azeotropic or heat-sensitive mixtures. Distillation relies on boiling point differences to separate components by vaporizing and condensing, requiring significant energy input and large equipment for effective separation. Your choice between pervaporation and distillation depends on factors like feed composition, thermal sensitivity, and energy costs in industrial applications.
Fundamental Principles of Pervaporation
Pervaporation relies on a selective membrane to separate components based on differences in their permeability and affinity, allowing one component to vaporize and pass through while the other remains liquid. This process operates under partial vapor pressure differences rather than relying on boiling point differences like distillation, enabling efficient separation of azeotropic mixtures or heat-sensitive compounds. Your choice of pervaporation offers energy savings and improved separation for complex mixtures that challenge traditional distillation techniques.
Core Mechanisms of Distillation
Distillation operates on the core mechanism of phase change, separating components based on differences in their boiling points through vaporization and condensation cycles. The process relies on heat to vaporize the more volatile component, which then condenses into a separate container, effectively purifying the mixture. Understanding this mechanism helps you optimize separation efficiency when choosing between pervaporation and distillation for your application.
Key Differences Between Pervaporation and Distillation
Pervaporation separates liquid mixtures using selective membranes that allow specific components to vaporize and pass through, driven by partial vapor pressure differences, while distillation relies on differences in boiling points to separate components by heating. Pervaporation operates efficiently at lower temperatures, reducing energy consumption and thermal degradation of heat-sensitive compounds, unlike distillation which often requires high energy input and elevated temperatures. The key differences include membrane selectivity and energy efficiency in pervaporation versus thermal phase change dependency in distillation.
Comparative Energy Consumption
Pervaporation consumes significantly less energy than distillation by selectively separating components through a membrane, reducing the need for phase change of the entire mixture. Distillation requires substantial heat input to vaporize and condense liquid mixtures, resulting in higher energy consumption especially for azeotropic or close-boiling components. Your process efficiency can be enhanced by choosing pervaporation when low-energy, selective separation is critical.
Applications in Industrial Separation Processes
Pervaporation offers energy-efficient separation of azeotropic mixtures and heat-sensitive compounds in chemical manufacturing and wastewater treatment, where traditional distillation faces limitations. Distillation remains the preferred method for large-scale separation of volatile components in petrochemical refining and alcohol production due to its scalability and well-established infrastructure. Your choice between pervaporation and distillation depends on factors such as feed composition, separation goals, and operational costs in industrial processes.
Selectivity and Efficiency: A Comparative Analysis
Pervaporation offers higher selectivity than distillation by using selective membranes that separate components based on their permeation rates, making it particularly efficient for separating azeotropic or close-boiling mixtures. Distillation relies on differences in volatility and often requires higher energy input, leading to lower efficiency in separating components with similar boiling points. Your choice between the two methods should consider the specific separation goals, with pervaporation providing superior selectivity and energy efficiency for targeted applications.
Environmental Impact and Sustainability Considerations
Pervaporation offers a lower environmental impact than distillation by consuming less energy and producing fewer greenhouse gas emissions due to its membrane-based separation process. Distillation requires significant heat input, often derived from fossil fuels, leading to higher carbon footprints and increased energy consumption. Implementing pervaporation can enhance sustainability in industrial separation by reducing water and energy usage, minimizing waste generation, and supporting eco-friendly solvent recovery.
Economic Factors: Cost of Operation and Scalability
Pervaporation offers lower operational costs due to reduced energy consumption compared to distillation, which requires substantial heat input for phase changes. Scalability of pervaporation systems benefits from modular design, enabling flexible capacity expansion with minimal capital investment. Your choice can optimize economic efficiency by balancing initial setup costs against long-term operational savings.
Future Trends and Innovations in Separation Technologies
Future trends in separation technologies emphasize the growing integration of pervaporation due to its energy efficiency and selectivity in separating azeotropic or close-boiling mixtures. Innovations focus on advanced membrane materials such as mixed-matrix membranes and nanocomposites that enhance permeation rates and durability. Your choice between pervaporation and distillation will increasingly depend on process optimization enabled by AI-driven control systems and sustainable energy use, driving lower environmental impact and operational costs.
pervaporation vs distillation Infographic
 libmatt.com
libmatt.com