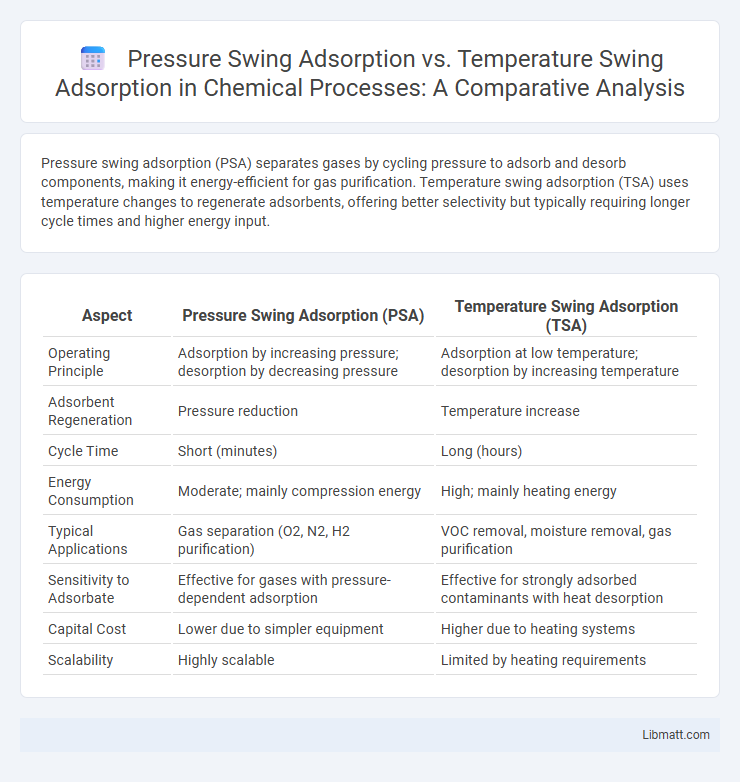
Pressure swing adsorption (PSA) separates gases by cycling pressure to adsorb and desorb components, making it energy-efficient for gas purification. Temperature swing adsorption (TSA) uses temperature changes to regenerate adsorbents, offering better selectivity but typically requiring longer cycle times and higher energy input.
Table of Comparison
| Aspect | Pressure Swing Adsorption (PSA) | Temperature Swing Adsorption (TSA) |
|---|---|---|
| Operating Principle | Adsorption by increasing pressure; desorption by decreasing pressure | Adsorption at low temperature; desorption by increasing temperature |
| Adsorbent Regeneration | Pressure reduction | Temperature increase |
| Cycle Time | Short (minutes) | Long (hours) |
| Energy Consumption | Moderate; mainly compression energy | High; mainly heating energy |
| Typical Applications | Gas separation (O2, N2, H2 purification) | VOC removal, moisture removal, gas purification |
| Sensitivity to Adsorbate | Effective for gases with pressure-dependent adsorption | Effective for strongly adsorbed contaminants with heat desorption |
| Capital Cost | Lower due to simpler equipment | Higher due to heating systems |
| Scalability | Highly scalable | Limited by heating requirements |
Introduction to Gas Separation Technologies
Pressure Swing Adsorption (PSA) and Temperature Swing Adsorption (TSA) are advanced gas separation technologies used to purify and concentrate gases by selectively adsorbing target components onto solid adsorbents. PSA operates by cycling pressure levels to adsorb gases at high pressure and desorb them at low pressure, making it energy-efficient for oxygen and hydrogen production. Your choice between PSA and TSA depends on factors such as the specific gas mixture, required purity, and operational costs, as TSA cycles temperature changes for regeneration, often suitable for capturing gases like carbon dioxide in industrial processes.
Overview of Pressure Swing Adsorption (PSA)
Pressure Swing Adsorption (PSA) is a widely used gas separation technique leveraging the selective adsorption of gas molecules under high pressure on porous adsorbents like zeolites or activated carbon. Unlike Temperature Swing Adsorption (TSA), PSA operates primarily by cycling between high and low pressures to adsorb and desorb target gases without significant temperature changes, making it energy-efficient for applications such as oxygen generation and hydrogen purification. Your choice of PSA ensures rapid cycle times and lower thermal stress on materials, optimizing performance in industrial gas separation processes.
Overview of Temperature Swing Adsorption (TSA)
Temperature Swing Adsorption (TSA) is a gas separation technique that regenerates the adsorbent by increasing the temperature to desorb the adsorbed species, making it suitable for removing impurities like moisture or organic compounds from gas streams. Unlike Pressure Swing Adsorption (PSA), which relies on pressure changes to adsorb and desorb gases, TSA operates through thermal cycles to achieve selective adsorption and regeneration. Your selection of TSA depends on factors such as the adsorption capacity, regeneration energy requirements, and the specific gas mixture involved in the separation process.
Key Principles: PSA vs TSA
Pressure Swing Adsorption (PSA) separates gases by cyclically increasing and decreasing pressure to preferentially adsorb specific components onto a solid adsorbent, typically zeolites or activated carbon. Temperature Swing Adsorption (TSA) utilizes temperature changes to regenerate the adsorbent, relying on different adsorption capacities at varying temperatures to separate gas mixtures. PSA is favored for faster cycle times and pressure-driven selectivity, while TSA offers advantages in handling contaminants with strong adsorption by leveraging thermal desorption.
Adsorbent Materials Comparison
Pressure swing adsorption (PSA) typically uses zeolites or activated carbon as adsorbent materials due to their high selectivity and adsorption capacity at varying pressures. Temperature swing adsorption (TSA) often employs materials like silica gel, activated alumina, or metal-organic frameworks that are effective at adsorption and desorption through temperature changes. Your choice between PSA and TSA depends on the adsorbents' thermal stability, regeneration efficiency, and operational conditions suited for your specific gas separation needs.
Process Efficiency and Performance
Pressure swing adsorption (PSA) achieves higher process efficiency by rapidly cycling pressure to selectively adsorb and desorb gases, resulting in faster separation and lower energy consumption compared to temperature swing adsorption (TSA). TSA relies on heating and cooling cycles, which typically demand more energy and time, reducing overall process performance, especially in large-scale or continuous applications. PSA systems offer superior gas purity and recovery rates due to their quicker response times and efficient pressure management.
Energy Consumption Analysis
Pressure Swing Adsorption (PSA) typically consumes less energy compared to Temperature Swing Adsorption (TSA) due to its reliance on pressure changes rather than temperature fluctuations to regenerate adsorbents. TSA requires significant thermal input to heat and cool the adsorbent beds, leading to higher energy demands and longer cycle times. Your choice between PSA and TSA should consider the specific energy efficiency requirements of your gas separation process.
Industrial Applications of PSA and TSA
Pressure Swing Adsorption (PSA) is extensively used in industrial gas separation processes such as oxygen generation, nitrogen production, and hydrogen purification due to its rapid cycle times and energy efficiency. Temperature Swing Adsorption (TSA) finds application in industries requiring removal of impurities like moisture or VOCs from air streams, commonly employed in natural gas processing and environmental control systems where adsorption capacity regenerates through temperature variation. PSA offers cost-effective, continuous operation suitable for large-scale gas production, while TSA provides higher purity levels for specific contaminants with longer regeneration cycles.
Advantages and Limitations
Pressure swing adsorption (PSA) offers rapid cycle times and energy efficiency by operating at near-ambient temperatures, making it ideal for gas separation in industries such as oxygen generation. In contrast, temperature swing adsorption (TSA) excels in removing trace contaminants due to its ability to regenerate adsorbents at elevated temperatures, though it involves longer cycle durations and higher energy consumption. Your choice between PSA and TSA depends on the specific application requirements, including gas composition, purity levels, and operational costs.
Choosing the Right Adsorption Method
Choosing the right adsorption method depends on the specific gas separation requirements and operational conditions of your process. Pressure Swing Adsorption (PSA) is ideal for applications needing rapid cycling and high-purity gas production, utilizing pressure changes to separate components effectively. Temperature Swing Adsorption (TSA) suits scenarios requiring adsorption of low-concentration contaminants, relying on temperature variations for regeneration and is better for bulk gas treatments or where thermal energy is readily available.
Pressure swing adsorption vs temperature swing adsorption Infographic
 libmatt.com
libmatt.com