A catalyst speeds up a chemical reaction by lowering the activation energy without being consumed, whereas an inhibitor slows down or prevents the reaction by interfering with the reactants or catalysts. Understanding the role of a catalyst or inhibitor is crucial for optimizing your chemical processes and improving efficiency.
Table of Comparison
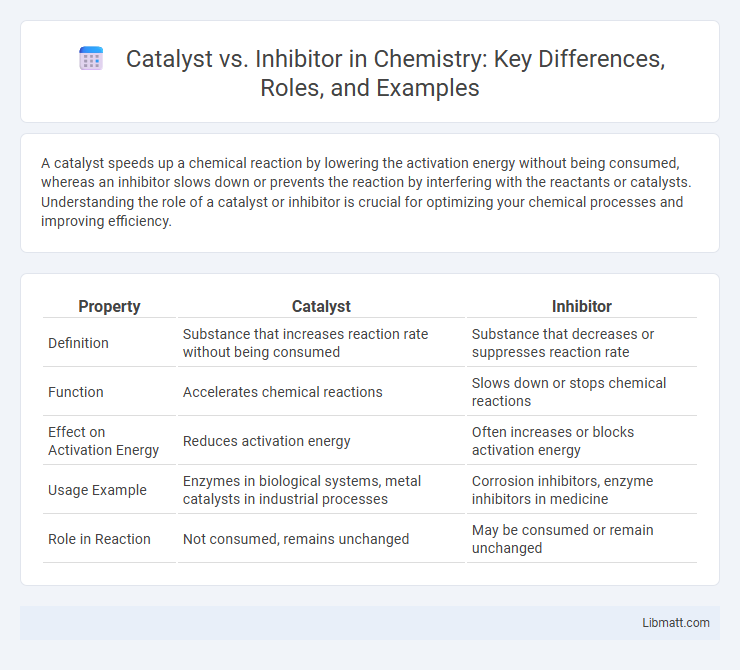
| Property | Catalyst | Inhibitor |
|---|---|---|
| Definition | Substance that increases reaction rate without being consumed | Substance that decreases or suppresses reaction rate |
| Function | Accelerates chemical reactions | Slows down or stops chemical reactions |
| Effect on Activation Energy | Reduces activation energy | Often increases or blocks activation energy |
| Usage Example | Enzymes in biological systems, metal catalysts in industrial processes | Corrosion inhibitors, enzyme inhibitors in medicine |
| Role in Reaction | Not consumed, remains unchanged | May be consumed or remain unchanged |
Understanding Catalysts: Definition and Role
Catalysts accelerate chemical reactions by lowering the activation energy, enabling processes to proceed faster without being consumed. Their role is crucial in both industrial applications and biological systems, as they enhance efficiency and selectivity in reactions. You can optimize reactions by selecting the appropriate catalyst tailored to your specific chemical process needs.
What Are Inhibitors? Key Characteristics
Inhibitors are substances that decrease the rate of a chemical reaction by interfering with the reactants or catalyst active sites. They bind to catalysts or reactants, reducing the efficiency of the catalytic process without being consumed in the reaction. Key characteristics of inhibitors include reversibility, specificity, and the ability to regulate reaction speed, making them crucial in controlling industrial and biochemical processes.
Fundamental Differences Between Catalysts and Inhibitors
Catalysts accelerate chemical reactions by lowering the activation energy, allowing reactants to convert into products faster without being consumed in the process. Inhibitors, on the other hand, slow down or prevent chemical reactions by interfering with reactants or catalysts, effectively increasing the activation energy. Your understanding of these fundamental differences is crucial for controlling reaction rates in industrial, biological, and environmental processes.
Mechanisms of Action: How Catalysts Work
Catalysts accelerate chemical reactions by lowering the activation energy barrier, enabling reactants to convert into products more efficiently without being consumed in the process. They achieve this by providing an alternative reaction pathway with a more favorable energy profile, often through the formation of transient intermediate complexes. Unlike inhibitors, which decrease reaction rates by interfering with reactants or catalysts, catalysts enhance reaction kinetics through precise molecular interactions and stabilization of transition states.
Mechanisms of Action: How Inhibitors Work
Inhibitors reduce the rate of chemical reactions by binding to enzymes and blocking substrate access or altering enzyme structure through competitive, non-competitive, or uncompetitive inhibition mechanisms. Competitive inhibitors resemble the substrate and compete for the active site, preventing substrate binding. Non-competitive inhibitors bind to allosteric sites, changing the enzyme's shape and decreasing catalytic activity without affecting substrate binding directly.
Real-Life Examples of Catalysts
Catalysts accelerate chemical reactions by lowering activation energy without being consumed, exemplified by enzymes in human digestion that speed up the breakdown of food molecules. Industrial processes utilize catalysts such as platinum in catalytic converters to reduce vehicle emissions by converting harmful gases into less toxic substances. In everyday life, yeast acts as a catalyst in baking by facilitating the fermentation process that causes dough to rise.
Common Applications of Inhibitors
Inhibitors are commonly used in corrosion prevention to slow down or stop chemical reactions that degrade metals, protecting pipelines and industrial equipment. They also play a critical role in controlling unwanted polymerization in manufacturing processes, ensuring product stability and quality. Your ability to manage reaction rates effectively with inhibitors enhances safety and efficiency in various chemical and industrial applications.
Impact on Reaction Rate: Catalysts vs Inhibitors
Catalysts increase the reaction rate by lowering the activation energy, enabling reactants to convert into products more efficiently without being consumed. Inhibitors decrease the reaction rate by interfering with the catalytic process or blocking active sites, thereby slowing down or halting the reaction. Understanding the distinct roles of catalysts and inhibitors is crucial for optimizing industrial chemical reactions and controlling biochemical pathways.
Industrial and Biological Significance
Catalysts accelerate chemical reactions by lowering activation energy, enabling efficient industrial processes such as petroleum refining and pharmaceuticals manufacturing. Inhibitors slow or halt reactions to prevent unwanted side effects like corrosion in pipelines and spoilage in food preservation. Both catalysts and inhibitors play crucial roles in biological systems, regulating metabolic pathways and ensuring cellular homeostasis.
Choosing Between a Catalyst and an Inhibitor
Choosing between a catalyst and an inhibitor depends on the desired effect on a chemical reaction; catalysts increase reaction rates by lowering activation energy, while inhibitors slow down or prevent reactions by interfering with reactants or catalytic sites. In industrial processes, catalysts are selected to enhance efficiency and yield, often using materials like metals or enzymes tailored to specific reactions, whereas inhibitors serve to control or halt unwanted reactions, such as corrosion or polymerization. Understanding the reaction mechanism, environmental conditions, and target outcomes guides the decision to employ either catalysts or inhibitors for optimal process control.
catalyst vs inhibitor Infographic
 libmatt.com
libmatt.com