Diazotization is a chemical reaction that converts primary aromatic amines into diazonium salts, essential for synthesizing azo dyes and other aromatic compounds, while nitrosation typically involves introducing a nitroso group into secondary amines or other nucleophiles, forming nitrosamines or nitrosamides. Understanding the difference between diazotization and nitrosation helps you optimize reaction conditions for desired chemical transformations in organic synthesis.
Table of Comparison
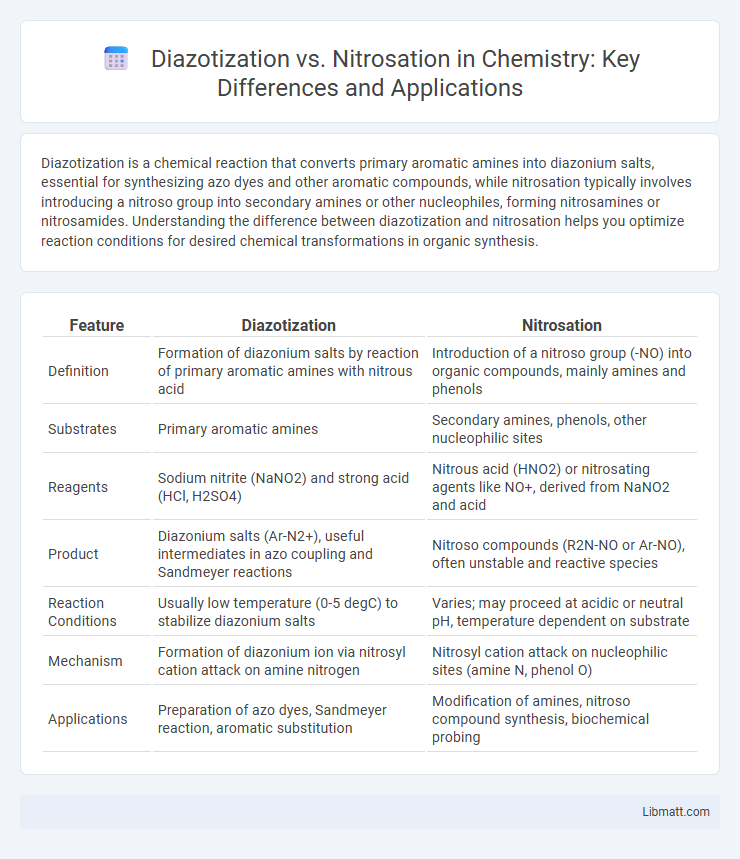
| Feature | Diazotization | Nitrosation |
|---|---|---|
| Definition | Formation of diazonium salts by reaction of primary aromatic amines with nitrous acid | Introduction of a nitroso group (-NO) into organic compounds, mainly amines and phenols |
| Substrates | Primary aromatic amines | Secondary amines, phenols, other nucleophilic sites |
| Reagents | Sodium nitrite (NaNO2) and strong acid (HCl, H2SO4) | Nitrous acid (HNO2) or nitrosating agents like NO+, derived from NaNO2 and acid |
| Product | Diazonium salts (Ar-N2+), useful intermediates in azo coupling and Sandmeyer reactions | Nitroso compounds (R2N-NO or Ar-NO), often unstable and reactive species |
| Reaction Conditions | Usually low temperature (0-5 degC) to stabilize diazonium salts | Varies; may proceed at acidic or neutral pH, temperature dependent on substrate |
| Mechanism | Formation of diazonium ion via nitrosyl cation attack on amine nitrogen | Nitrosyl cation attack on nucleophilic sites (amine N, phenol O) |
| Applications | Preparation of azo dyes, Sandmeyer reaction, aromatic substitution | Modification of amines, nitroso compound synthesis, biochemical probing |
Introduction to Diazotization and Nitrosation
Diazotization involves converting aromatic primary amines into diazonium salts, primarily used in azo dye synthesis, while nitrosation refers to introducing a nitroso group (-NO) into amines or other compounds, often producing nitrosamines. Your understanding of diazotization is crucial in organic chemistry for creating versatile intermediates, whereas nitrosation plays a significant role in modifying molecular structures for pharmaceutical and industrial applications. Both reactions utilize nitrous acid but differ in substrates and mechanisms, influencing product formation and reaction conditions.
Chemical Definitions: Diazotization vs Nitrosation
Diazotization is a chemical reaction in which a primary aromatic amine reacts with nitrous acid to form a diazonium salt, primarily used in the synthesis of azo dyes and aromatic substitutions. Nitrosation involves the introduction of a nitroso group (-NO) into a molecule via nitrosating agents, commonly targeting secondary amines or phenols to produce nitrosamines or nitrosophenols. Both processes involve nitrous acid derivatives but differ fundamentally in their substrates and reaction products.
Mechanisms of Diazotization
Diazotization involves the reaction of primary aromatic amines with nitrous acid, producing diazonium salts through the formation of nitrosamines as key intermediates. The mechanism initiates with the protonation of nitrous acid to generate the nitrosonium ion (NO+), which electrophilically attacks the amine nitrogen, forming an N-nitrosamine. Subsequent proton transfers and loss of water lead to the diazonium ion, a versatile intermediate in synthetic organic chemistry used for azo coupling and Sandmeyer reactions.
Mechanisms of Nitrosation
Nitrosation involves the substitution or addition of a nitroso group (-NO) to nucleophilic centers, typically amines, through the formation of nitrosating agents such as nitrous acid (HNO2) or its derivatives. The mechanism proceeds via the generation of electrophilic nitrosonium ions (NO+), which react with nucleophiles leading to N-nitrosamines or other nitroso compounds depending on substrate structure. Your understanding of nitrosation's electrophilic attack and intermediate formation is critical for differentiating it from diazotization, where diazonium salts are primarily formed from primary aromatic amines under acidic conditions.
Key Reactants and Conditions
Diazotization primarily involves primary aromatic amines reacting with nitrous acid (HNO2) under acidic conditions, typically at low temperatures (0-5degC) to form diazonium salts. Nitrosation targets secondary amines, where nitrous acid or nitrosonium ions react under acidic or mildly acidic conditions, generating nitrosamines. Control of pH and temperature is crucial in both reactions to ensure selective formation of diazonium compounds or nitrosamines without side reactions.
Comparative Reaction Pathways
Diazotization involves the formation of diazonium salts from primary aromatic amines through reaction with nitrous acid under acidic conditions, characterized by the initial formation of a diazonium ion intermediate. Nitrosation typically refers to the introduction of a nitroso group (-NO) into organic substrates, such as secondary amines, via electrophilic attack by nitrosonium ions, resulting in nitrosamines. The key distinction lies in diazotization generating diazonium compounds via nucleophilic substitution, whereas nitrosation predominantly proceeds through electrophilic substitution or addition mechanisms.
Industrial and Laboratory Applications
Diazotization is widely used in the synthesis of azo dyes, pharmaceuticals, and agrochemicals due to its ability to form stable diazonium salts under controlled acidic conditions. Nitrosation finds significant industrial application in the production of nitroso compounds, such as rubber vulcanization accelerators and pharmaceuticals, through the reaction of nitrous acid with secondary amines. In laboratories, diazotization enables the preparation of reactive intermediates for electrophilic aromatic substitution, while nitrosation is valuable for studying nitrosamine formation and its implications in toxicology.
Safety Considerations and Toxicity
Diazotization involves the formation of diazonium salts, which can be highly reactive and potentially explosive, requiring strict temperature control and the use of fume hoods to minimize exposure to toxic nitrogen oxides. Nitrosation generates nitrosamines, compounds known for their carcinogenicity, demanding careful handling, appropriate personal protective equipment, and rigorous waste disposal protocols to protect your health. Both processes require stringent safety measures to prevent inhalation, skin contact, and environmental contamination due to their inherent toxicity risks.
Analytical Methods for Product Identification
Analytical methods for identifying products of diazotization primarily include UV-Vis spectroscopy and nuclear magnetic resonance (NMR) spectroscopy, which detect characteristic diazonium salt signals and confirm molecular structure. Gas chromatography-mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC) coupled with UV or MS detectors are widely used to analyze nitrosation products, providing detailed molecular mass and fragmentation patterns. Infrared (IR) spectroscopy also aids in distinguishing functional groups formed during diazotization and nitrosation, enhancing product characterization.
Summary of Differences and Similarities
Diazotization involves the reaction of primary aromatic amines with nitrous acid to form diazonium salts, primarily used in synthesizing azo dyes and aromatic compounds, while nitrosation introduces a nitroso group into amines or other nucleophiles, producing nitrosamines or nitrosamides, often relevant in biological and industrial contexts. Both processes rely on nitrous acid as a key reagent and share mechanistic steps involving electrophilic nitrosyl species, but diazotization is specific to primary aromatic amines and typically occurs under acidic conditions, whereas nitrosation can occur with various amines under a broader range of conditions. Your understanding of these reactions enhances the ability to manipulate nitrogen-containing functional groups in synthetic chemistry and medical research.
Diazotization vs nitrosation Infographic
 libmatt.com
libmatt.com