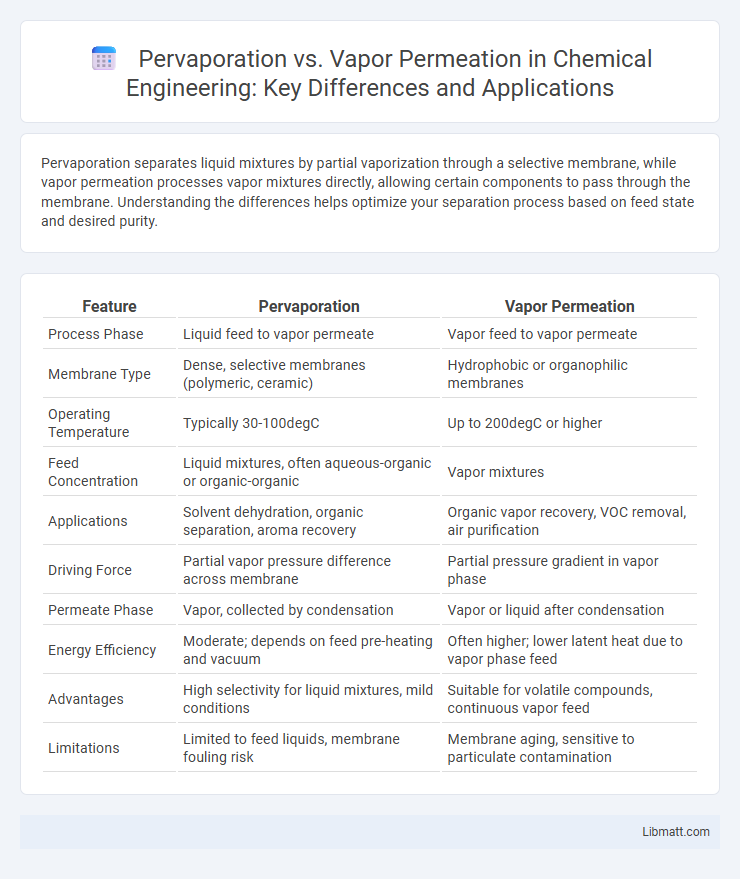
Pervaporation separates liquid mixtures by partial vaporization through a selective membrane, while vapor permeation processes vapor mixtures directly, allowing certain components to pass through the membrane. Understanding the differences helps optimize your separation process based on feed state and desired purity.
Table of Comparison
| Feature | Pervaporation | Vapor Permeation |
|---|---|---|
| Process Phase | Liquid feed to vapor permeate | Vapor feed to vapor permeate |
| Membrane Type | Dense, selective membranes (polymeric, ceramic) | Hydrophobic or organophilic membranes |
| Operating Temperature | Typically 30-100degC | Up to 200degC or higher |
| Feed Concentration | Liquid mixtures, often aqueous-organic or organic-organic | Vapor mixtures |
| Applications | Solvent dehydration, organic separation, aroma recovery | Organic vapor recovery, VOC removal, air purification |
| Driving Force | Partial vapor pressure difference across membrane | Partial pressure gradient in vapor phase |
| Permeate Phase | Vapor, collected by condensation | Vapor or liquid after condensation |
| Energy Efficiency | Moderate; depends on feed pre-heating and vacuum | Often higher; lower latent heat due to vapor phase feed |
| Advantages | High selectivity for liquid mixtures, mild conditions | Suitable for volatile compounds, continuous vapor feed |
| Limitations | Limited to feed liquids, membrane fouling risk | Membrane aging, sensitive to particulate contamination |
Introduction to Pervaporation and Vapor Permeation
Pervaporation and vapor permeation are advanced membrane separation techniques commonly used for liquid mixture separation and gas purification. Pervaporation involves the selective evaporation of a liquid mixture through a dense membrane, driven by a partial pressure difference, effectively separating components based on their volatility and affinity to the membrane material. Vapor permeation, on the other hand, separates vapor mixtures by allowing selective permeation of certain gaseous components through a membrane, facilitating applications such as air dehydration and solvent recovery.
Fundamental Principles of Each Separation Process
Pervaporation separates liquid mixtures by selectively permeating components through a dense, non-porous membrane, where the permeate evaporates on the downstream side under reduced pressure or sweeping gas conditions. Vapor permeation involves the selective transport of vapor-phase components through a membrane driven by partial pressure differences, without phase change on the feed side. Both processes rely on differences in solubility and diffusivity of molecules in the membrane material, but pervaporation integrates a liquid-to-vapor phase transition, while vapor permeation operates entirely in the vapor phase.
Key Differences Between Pervaporation and Vapor Permeation
Pervaporation separates liquid mixtures by partial vaporization through a non-porous membrane, whereas vapor permeation involves the selective passage of vapor molecules through a membrane from one vapor phase to another. Pervaporation is commonly used for separating azeotropic mixtures and removing trace solvents, while vapor permeation is applied in gas dehydration and volatile organic compound removal. The main differences lie in their feed phases, membrane types, and operational applications, with pervaporation handling liquid feeds and vapor permeation processing vapor feeds.
Membrane Materials: Selection and Performance
Pervaporation membranes typically utilize hydrophilic or organophilic polymeric materials such as polyvinyl alcohol (PVA) and polydimethylsiloxane (PDMS), optimized for selective permeation of liquid mixtures. Vapor permeation membranes often employ dense, non-porous materials like silicone or composite membranes designed to separate vapor phase components based on condensability and diffusivity differences. Material selection directly influences separation performance, permeability, and selectivity, with thermal and chemical stability being critical for operational efficiency in both processes.
Process Mechanisms and Operating Conditions
Pervaporation separates mixtures by selective permeation through a dense membrane followed by vaporization on the permeate side under reduced pressure or sweeping gas, optimizing separation at moderate temperatures typically between 30-80degC. Vapor permeation also uses membranes but operates under higher temperatures, often 100-200degC, relying on partial vaporization of feed components before membrane transport driven by a partial pressure gradient. Your process choice depends on feed composition and temperature tolerance, with pervaporation suited for liquid feed separation and vapor permeation for vapor-phase mixtures requiring higher thermal resistance.
Applications in Industry: A Comparative Overview
Pervaporation is widely utilized in the chemical and pharmaceutical industries for separating azeotropic mixtures and removing organic solvents from water due to its high selectivity and energy efficiency. Vapor permeation finds extensive applications in natural gas dehydration and volatile organic compound (VOC) recovery because it excels in handling gas mixtures and high-volume fluxes. Both technologies serve critical roles in environmental protection and process optimization, with pervaporation favored for liquid separations and vapor permeation preferred for gas-phase separations.
Advantages and Limitations of Pervaporation
Pervaporation offers high selectivity and energy efficiency for separating liquid mixtures, especially for azeotropic or close-boiling components. It allows operation at lower temperatures, reducing thermal degradation risks and enabling the treatment of heat-sensitive compounds. However, your process may face limitations such as membrane fouling, limited membrane lifespan, and scale-up challenges for large industrial applications.
Benefits and Challenges of Vapor Permeation
Vapor permeation offers significant benefits such as high selectivity and energy efficiency in separating vapor mixtures, making it ideal for applications like solvent recovery and dehydration. It enables continuous operation with a flexible design for different feed compositions, but challenges include membrane fouling, sensitivity to feed contaminants, and limited mechanical strength under harsh industrial conditions. Optimizing your vapor permeation system requires careful material selection and maintenance to maximize performance and durability.
Recent Advances and Innovations in Membrane Technology
Recent advances in membrane technology have enhanced both pervaporation and vapor permeation processes by developing novel polymeric and mixed-matrix membranes with superior selectivity and permeability. Innovations in nano-structured materials and surface modifications have improved membrane stability and separation efficiency, enabling more energy-efficient and cost-effective applications for organic solvent dehydration and air purification. Your choice of membrane technology can now leverage these cutting-edge developments to optimize performance in industrial gas separation and liquid mixture processing.
Future Perspectives and Market Trends
Pervaporation and vapor permeation technologies are gaining traction in membrane separation markets due to increasing demand for energy-efficient and sustainable processes in chemical and environmental industries. Innovations in membrane materials, such as mixed matrix and graphene-based membranes, enhance selectivity and permeability, driving adoption in biofuel production, solvent recovery, and water treatment sectors. Market trends indicate robust growth fueled by tightening environmental regulations, escalating industrial wastewater challenges, and rising investments in green technologies.
Pervaporation vs vapor permeation Infographic
 libmatt.com
libmatt.com