Propylene oxide is commonly used in the production of polyurethanes and is less volatile with a slightly higher boiling point compared to ethylene oxide, which serves as a powerful sterilizing agent and a precursor for antifreeze and detergents. Understanding your specific application helps determine which compound offers the best balance of reactivity and safety for industrial or medical uses.
Table of Comparison
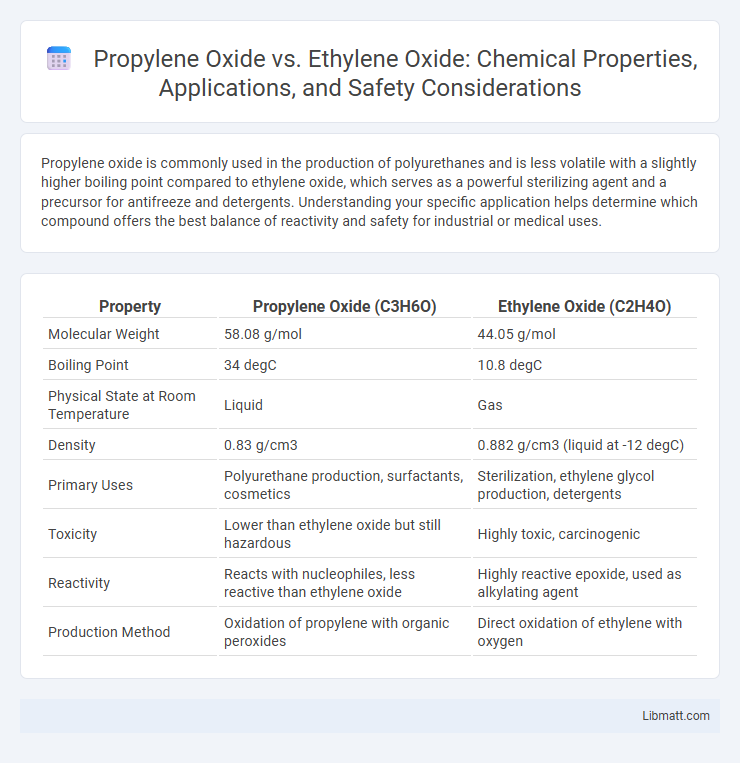
| Property | Propylene Oxide (C3H6O) | Ethylene Oxide (C2H4O) |
|---|---|---|
| Molecular Weight | 58.08 g/mol | 44.05 g/mol |
| Boiling Point | 34 degC | 10.8 degC |
| Physical State at Room Temperature | Liquid | Gas |
| Density | 0.83 g/cm3 | 0.882 g/cm3 (liquid at -12 degC) |
| Primary Uses | Polyurethane production, surfactants, cosmetics | Sterilization, ethylene glycol production, detergents |
| Toxicity | Lower than ethylene oxide but still hazardous | Highly toxic, carcinogenic |
| Reactivity | Reacts with nucleophiles, less reactive than ethylene oxide | Highly reactive epoxide, used as alkylating agent |
| Production Method | Oxidation of propylene with organic peroxides | Direct oxidation of ethylene with oxygen |
Introduction to Propylene Oxide and Ethylene Oxide
Propylene oxide and ethylene oxide are two essential epoxides widely used in the chemical industry for synthesizing various products. Propylene oxide primarily serves as a precursor in the manufacture of polyurethanes, glycols, and surfactants, while ethylene oxide is crucial for producing ethylene glycol, detergents, and sterilizing agents. Both compounds are valued for their reactivity and versatility in producing solvents, plastics, and antifreeze formulations.
Chemical Structure and Properties Comparison
Propylene oxide (C3H6O) consists of a three-membered oxirane ring with a methyl group attached, resulting in slightly higher molecular weight and boiling point compared to ethylene oxide (C2H4O), which has a simpler three-membered oxirane ring without substituents. The presence of the methyl group in propylene oxide imparts increased hydrophobicity and boiling point (~34degC) versus ethylene oxide's boiling point (~10.7degC), influencing their reactivity and applications. Both compounds are highly reactive epoxides, but ethylene oxide is more volatile and water-soluble, while propylene oxide's structure makes it more chemically stable and less hazardous to handle.
Industrial Production Methods
Propylene oxide is primarily produced through the chlorohydrin process or by oxidation using organic peroxides, while ethylene oxide is mainly manufactured via the direct oxidation of ethylene over a silver catalyst. The chlorohydrin method for propylene oxide involves reacting propylene with chlorine and water, generating propylene chlorohydrins that are subsequently converted to propylene oxide. In contrast, ethylene oxide production relies on an efficient catalytic process that combines ethylene and oxygen under controlled temperature and pressure, resulting in a highly selective and industrially scalable method.
Applications in Various Industries
Propylene oxide is extensively used in the production of polyether polyols for flexible and rigid foams in the automotive, construction, and furniture industries, while ethylene oxide is primarily utilized for sterilizing medical equipment and producing glycol-based antifreeze and detergents. Your choice between these chemicals depends on whether you need a versatile intermediate for manufacturing or a potent sterilizing agent. Both play critical roles in sectors ranging from healthcare to manufacturing, highlighting their industrial significance.
Safety and Handling Considerations
Propylene oxide and ethylene oxide both require stringent safety protocols due to their flammability, toxicity, and carcinogenic properties; however, ethylene oxide poses higher acute toxicity and greater explosive hazard, necessitating more rigorous ventilation and explosion-proof equipment. Proper personal protective equipment (PPE), continuous monitoring for leaks, and adherence to exposure limits set by agencies like OSHA and NIOSH are critical for handling both chemicals safely. Storage in cool, well-ventilated areas away from sources of ignition minimizes risk, with ethylene oxide demanding stricter controls due to its lower explosive limits and higher reactivity.
Environmental Impact and Regulations
Propylene oxide and ethylene oxide differ significantly in environmental impact and regulatory treatment due to their chemical properties and uses. Ethylene oxide is classified as a carcinogen and tightly regulated by agencies like the EPA and OSHA, requiring stringent emission controls to minimize air and water pollution. Your industrial processes must comply with these regulations, while propylene oxide, considered less hazardous, faces fewer restrictions but still requires careful handling to reduce volatile organic compound (VOC) emissions and ensure environmental safety.
Toxicity and Health Risks
Propylene oxide presents lower acute toxicity compared to ethylene oxide but remains a probable human carcinogen with risks of respiratory irritation and central nervous system effects. Ethylene oxide is classified as a known human carcinogen linked to increased risks of leukemia and lymphoma, causing severe respiratory issues and reproductive hazards. Both chemicals require strict exposure limits and protective measures to mitigate adverse health effects in industrial and medical settings.
Economic Factors and Market Trends
Propylene oxide commands a higher market value than ethylene oxide due to its extensive use in polyurethanes and propylene glycol production, driving significant demand growth. Ethylene oxide benefits from broader applications in sterilization and antifreeze manufacturing, sustaining steady global market expansion despite lower pricing. Shifts in raw material availability and environmental regulations are influencing the cost dynamics and investment patterns within both markets, impacting long-term profitability and supply chain strategies.
Advances in Sustainable Alternatives
Propylene oxide offers a lower environmental impact compared to ethylene oxide due to its reduced toxicity and lower greenhouse gas emissions during production. Recent advances in sustainable alternatives emphasize bio-based propylene oxide derived from renewable feedstocks like glycerol, which enhances circular economy efforts. Innovations in catalytic processes also improve energy efficiency and reduce waste generation, making propylene oxide a key candidate for greener chemical manufacturing.
Future Outlook and Innovations
Emerging innovations in propylene oxide production focus on sustainable processes such as bio-based feedstocks and energy-efficient catalytic pathways, enhancing environmental compatibility. Ethylene oxide advancements prioritize safer handling techniques and alternative synthesis methods to reduce toxic emissions and improve worker safety in industrial settings. The future outlook for both chemicals hinges on integrating green chemistry principles and digital technologies to optimize production efficiency and reduce carbon footprints.
Propylene oxide vs ethylene oxide Infographic
 libmatt.com
libmatt.com