Sintering involves heating powders just below their melting points to bond particles and increase material density, enhancing strength and durability, while calcination refers to heating materials at high temperatures in the absence of air or oxygen to cause thermal decomposition, remove volatile substances, or induce phase transitions. Understanding the differences between sintering and calcination is crucial for optimizing Your manufacturing processes in ceramics, metallurgy, and catalyst production.
Table of Comparison
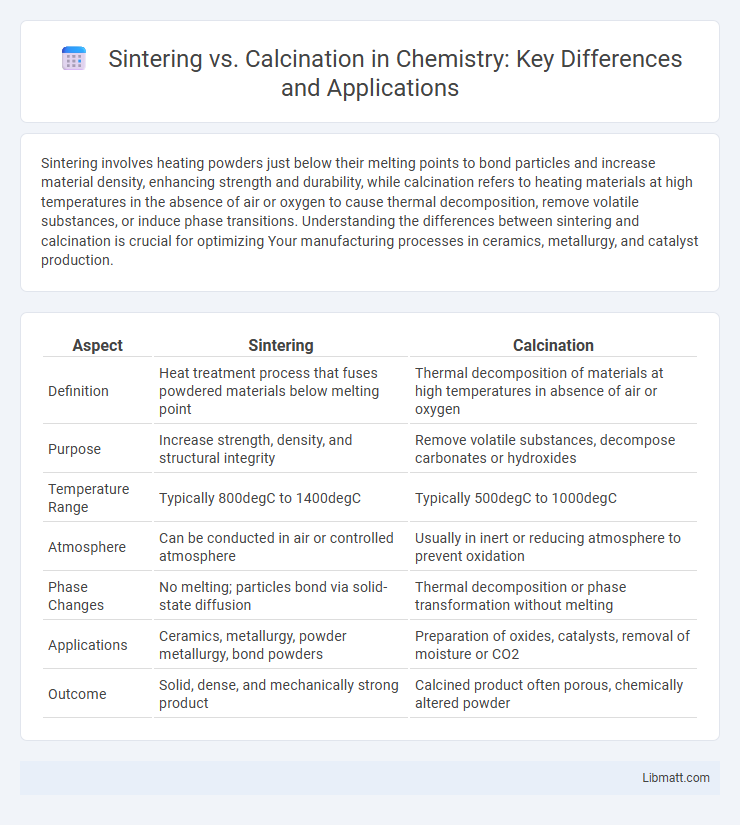
| Aspect | Sintering | Calcination |
|---|---|---|
| Definition | Heat treatment process that fuses powdered materials below melting point | Thermal decomposition of materials at high temperatures in absence of air or oxygen |
| Purpose | Increase strength, density, and structural integrity | Remove volatile substances, decompose carbonates or hydroxides |
| Temperature Range | Typically 800degC to 1400degC | Typically 500degC to 1000degC |
| Atmosphere | Can be conducted in air or controlled atmosphere | Usually in inert or reducing atmosphere to prevent oxidation |
| Phase Changes | No melting; particles bond via solid-state diffusion | Thermal decomposition or phase transformation without melting |
| Applications | Ceramics, metallurgy, powder metallurgy, bond powders | Preparation of oxides, catalysts, removal of moisture or CO2 |
| Outcome | Solid, dense, and mechanically strong product | Calcined product often porous, chemically altered powder |
Introduction to Sintering and Calcination
Sintering and calcination are essential thermal processes used in material science and industrial manufacturing to alter the physical and chemical properties of raw materials. Sintering involves heating powdered materials below their melting point to create a solid mass through particle bonding, while calcination refers to the thermal decomposition or removal of volatile substances from a material, often resulting in phase changes or material purification. Your choice between sintering and calcination depends on the desired strength, porosity, or chemical composition of the final product.
Defining Sintering: Process and Purpose
Sintering is a high-temperature process used to compact and form solid materials from powders by heating them below their melting point, causing particle bonding through diffusion and coalescence. This process enhances mechanical strength, structural integrity, and densification of ceramics, metals, and composites, making it crucial in manufacturing industries like metallurgy and additive manufacturing. Unlike calcination, which primarily involves thermal decomposition and removal of volatile substances, sintering focuses on solid-state diffusion to produce a dense, cohesive mass.
Understanding Calcination: Key Principles
Calcination is a thermal treatment process that decomposes material by heating it to high temperatures in the absence or limited supply of air, primarily to remove volatile substances and induce phase transformations. This process is essential in industries such as cement production, metallurgy, and ceramics to drive off carbon dioxide from carbonates and improve material properties. Unlike sintering, which fuses particles together by heating below the melting point, calcination focuses on chemical decomposition without melting the material.
Major Differences Between Sintering and Calcination
Sintering involves heating powdered materials below their melting point to create a solid mass through particle bonding, enhancing density and mechanical strength. Calcination is the thermal decomposition of materials at high temperatures, often leading to phase changes or removal of volatile substances without melting. Your choice depends on whether you need consolidation of particles (sintering) or chemical transformation and removal of impurities (calcination).
Temperature Ranges in Sintering vs Calcination
Sintering typically occurs at higher temperature ranges, often between 800degC and 1400degC, to facilitate the bonding of particles into a dense solid mass without melting. Calcination involves lower temperature ranges, generally from 500degC to 900degC, aimed at decomposing carbonates or removing volatile substances from raw materials. The distinct temperature windows reflect differences in phase transformations and chemical reactions essential to each process.
Common Materials Used in Both Processes
Sintering and calcination commonly involve materials such as metal oxides, ceramics, and minerals including alumina, silica, and limestone. During sintering, powders of these materials are heated below their melting point to form a dense solid, whereas calcination typically involves the thermal decomposition of carbonates or hydroxides, releasing gases and creating oxides. Your choice of material directly impacts the efficiency and outcome of each process in industrial applications.
Industrial Applications of Sintering
Sintering finds extensive industrial applications in manufacturing advanced ceramics, metal parts, and powder metallurgy components due to its ability to enhance material strength and density through controlled heating below melting points. Industries such as automotive, aerospace, and electronics rely on sintering to produce wear-resistant parts, magnets, and electronic substrates with precise dimensions and improved mechanical properties. This process is crucial for optimizing performance and reducing waste in mass production of complex, high-performance materials.
Industrial Applications of Calcination
Calcination is widely used in the cement industry to decompose limestone into lime and carbon dioxide, crucial for clinker production. In the metallurgy sector, calcination assists in removing volatile substances and moisture from ores, enhancing metal extraction efficiency. The chemical industry employs calcination to activate catalysts and purify raw materials by driving off impurities and altering material phases.
Advantages and Limitations of Each Process
Sintering offers advantages such as enhanced mechanical strength and improved material density by fusing particles at high temperatures without melting, but it requires precise temperature control and can lead to grain growth affecting material properties. Calcination efficiently removes volatile substances and induces phase transformations, making it ideal for preparing catalysts and removing moisture, yet it may cause particle agglomeration and structural changes unfavorable for some applications. Your choice between sintering and calcination depends on the desired material characteristics and process constraints, balancing the benefits of densification against potential microstructural alterations.
Conclusion: Choosing Sintering or Calcination
Choosing between sintering and calcination depends on the desired material properties and application requirements. Sintering involves compacting and forming solid materials at high temperatures to enhance density and mechanical strength, while calcination primarily drives off volatile substances or induces phase transitions without melting. Your decision should consider factors like temperature range, material phase changes, and end-use performance to achieve optimal results.
Sintering vs calcination Infographic
 libmatt.com
libmatt.com