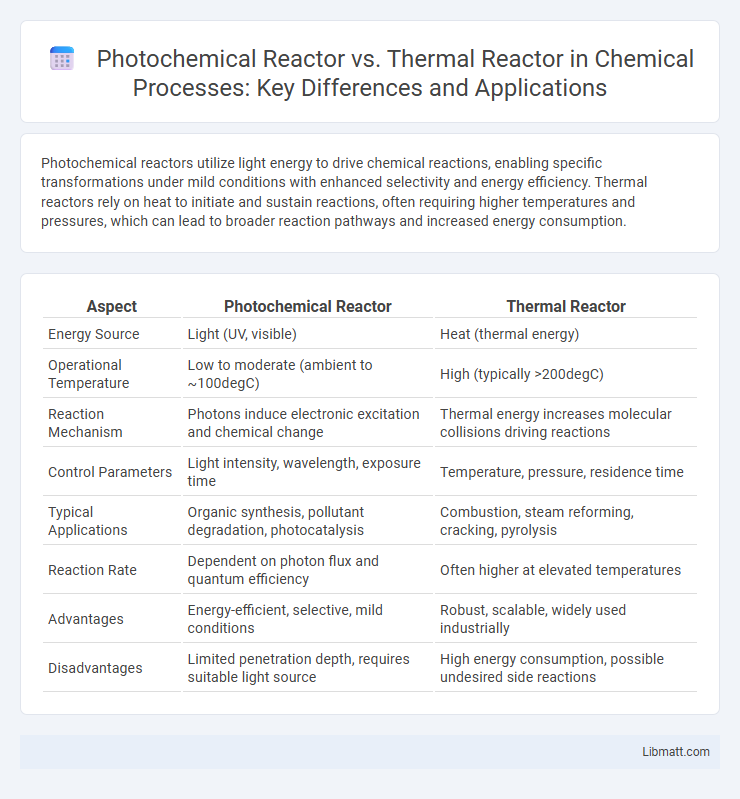
Photochemical reactors utilize light energy to drive chemical reactions, enabling specific transformations under mild conditions with enhanced selectivity and energy efficiency. Thermal reactors rely on heat to initiate and sustain reactions, often requiring higher temperatures and pressures, which can lead to broader reaction pathways and increased energy consumption.
Table of Comparison
| Aspect | Photochemical Reactor | Thermal Reactor |
|---|---|---|
| Energy Source | Light (UV, visible) | Heat (thermal energy) |
| Operational Temperature | Low to moderate (ambient to ~100degC) | High (typically >200degC) |
| Reaction Mechanism | Photons induce electronic excitation and chemical change | Thermal energy increases molecular collisions driving reactions |
| Control Parameters | Light intensity, wavelength, exposure time | Temperature, pressure, residence time |
| Typical Applications | Organic synthesis, pollutant degradation, photocatalysis | Combustion, steam reforming, cracking, pyrolysis |
| Reaction Rate | Dependent on photon flux and quantum efficiency | Often higher at elevated temperatures |
| Advantages | Energy-efficient, selective, mild conditions | Robust, scalable, widely used industrially |
| Disadvantages | Limited penetration depth, requires suitable light source | High energy consumption, possible undesired side reactions |
Introduction to Photochemical and Thermal Reactors
Photochemical reactors use light energy to drive chemical reactions, making them ideal for processes such as photocatalysis and UV-induced reactions, while thermal reactors rely on heat to initiate and sustain chemical transformations typically through combustion or high-temperature reactions. Photochemical reactors often employ lamps or lasers as light sources, enabling precise control over reaction pathways and energy efficiency. Your choice between these reactors depends on the specific reaction mechanism, energy requirements, and desired product selectivity.
Fundamental Principles of Photochemical Reactors
Photochemical reactors operate based on the fundamental principle of utilizing light energy, typically ultraviolet or visible light, to initiate and drive chemical reactions through photon absorption by reactants or catalysts. This process enables selective activation of molecular bonds, often leading to unique reaction pathways unavailable in thermal reactors, which rely solely on heat to increase reaction rates. Your choice between photochemical and thermal reactors depends on factors like reaction specificity, energy efficiency, and product selectivity.
Key Mechanisms in Thermal Reactors
Thermal reactors primarily rely on heat to initiate and sustain chemical reactions, leveraging high temperatures to increase molecular collisions and reaction rates. The key mechanisms include thermal activation of reactants, diffusion processes, and energy transfer through conduction or convection. Understanding these thermal dynamics allows you to optimize reactor conditions for improved efficiency and yield in industrial applications.
Energy Sources: Light vs Heat
Photochemical reactors rely on light energy, usually ultraviolet or visible light, to initiate and drive chemical reactions through photon absorption, making them highly efficient for processes requiring specific wavelength activation. Thermal reactors use heat as the primary energy source, providing the thermal energy needed to overcome activation barriers and sustain high-temperature reactions essential for traditional catalytic and combustion processes. Your choice between these reactors depends on the energy availability and reaction mechanisms, where photochemical reactors offer precise energy input via photons, while thermal reactors depend on a consistent heat supply to maintain reaction rates.
Reaction Pathways and Selectivity Differences
Photochemical reactors utilize light energy to initiate and control reaction pathways, enabling high selectivity through activation of specific molecules or intermediates that are inaccessible in thermal reactors. Thermal reactors rely on heat to drive reactions, often producing a broader range of products due to higher energy input causing non-selective bond cleavage. Consequently, photochemical reactors offer enhanced selectivity for desired products by exploiting wavelength-specific photon absorption, while thermal reactors tend to facilitate multiple competing pathways resulting in lower selectivity.
Efficiency and Yield Comparisons
Photochemical reactors utilize light energy to drive specific chemical reactions, often resulting in higher selectivity and yield for photochemically active compounds compared to thermal reactors, which rely on heat to initiate reactions. The efficiency of photochemical reactors is enhanced by their ability to operate under milder conditions and direct energy input, reducing side reactions and energy consumption relative to thermal reactors. Thermal reactors generally achieve faster reaction rates at higher temperatures but may suffer from lower selectivity and increased by-product formation, impacting overall yield and process efficiency.
Applications in Industry and Research
Photochemical reactors are extensively used in industries such as pharmaceuticals and environmental engineering for processes like photocatalytic degradation and selective organic synthesis, where light-driven reactions offer high specificity and mild operating conditions. Thermal reactors dominate applications in petrochemical refining and bulk chemical production by enabling high-temperature catalytic processes essential for cracking and reforming hydrocarbons efficiently. Your choice between these reactors depends on whether your focus is precise photochemical transformations or large-scale thermal conversions.
Advantages and Limitations: Photochemical vs Thermal
Photochemical reactors offer precise control of reaction pathways through light energy, enabling selective synthesis with lower temperatures and reduced energy consumption, while thermal reactors rely on heat, providing robust scalability and broad applicability for high-temperature processes. Photochemical systems are limited by light penetration depth and often require specialized light sources, whereas thermal reactors face challenges with energy efficiency and potential thermal degradation of sensitive compounds. Understanding these advantages and limitations helps you choose the optimal reactor type based on reaction specificity, energy considerations, and material compatibility.
Safety Considerations in Reactor Design
Photochemical reactors offer enhanced safety by operating at lower temperatures and pressures, reducing risks of thermal degradation and explosions compared to thermal reactors. Thermal reactors, with their high-temperature environments, require robust materials and cooling systems to manage potential hazards such as runaway reactions and equipment failure. Your choice in reactor design should prioritize safety protocols aligned with the specific reaction conditions and risk factors inherent to photochemical or thermal processes.
Future Trends in Reactor Technologies
Photochemical reactors are evolving with innovations in LED light sources and advanced photocatalysts to enhance energy efficiency and selectivity in chemical synthesis. Thermal reactors continue to improve through the integration of microreactor technology and advanced heat management systems, enabling precise temperature control and intensified reaction rates. Future reactor technologies emphasize hybrid designs combining photochemical and thermal processes to achieve sustainable and high-throughput chemical manufacturing.
Photochemical reactor vs thermal reactor Infographic
 libmatt.com
libmatt.com