Hydroformylation transforms alkenes into aldehydes by adding a formyl group and a hydrogen atom, expanding your options for synthesizing valuable intermediates in pharmaceuticals and plastics. Hydrogenation, on the other hand, saturates unsaturated bonds by adding hydrogen molecules, commonly used to convert alkenes into alkanes or reduce aromatics, enhancing stability and altering physical properties.
Table of Comparison
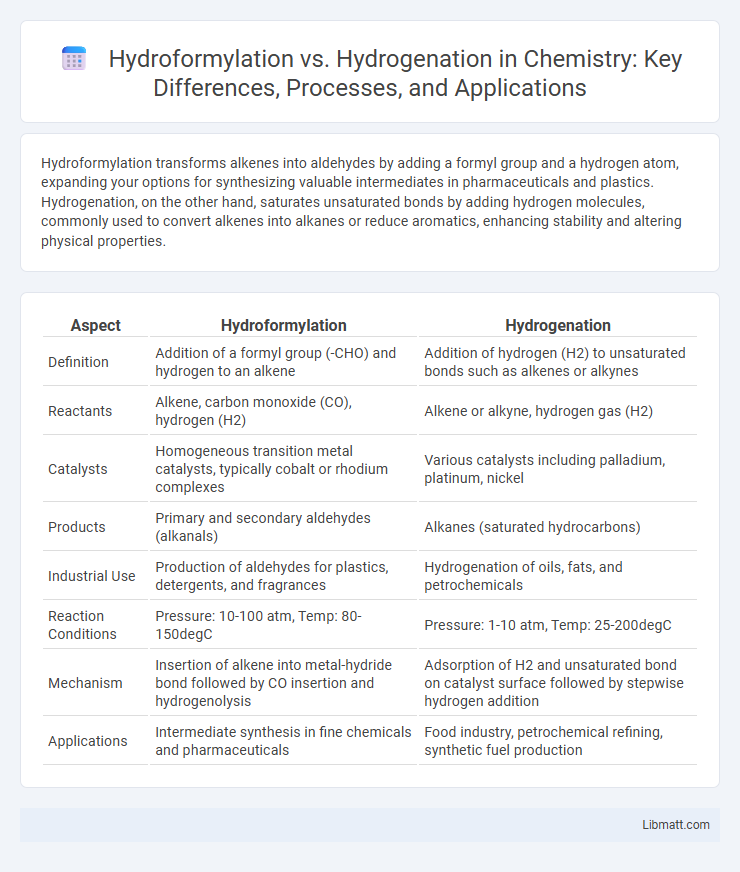
| Aspect | Hydroformylation | Hydrogenation |
|---|---|---|
| Definition | Addition of a formyl group (-CHO) and hydrogen to an alkene | Addition of hydrogen (H2) to unsaturated bonds such as alkenes or alkynes |
| Reactants | Alkene, carbon monoxide (CO), hydrogen (H2) | Alkene or alkyne, hydrogen gas (H2) |
| Catalysts | Homogeneous transition metal catalysts, typically cobalt or rhodium complexes | Various catalysts including palladium, platinum, nickel |
| Products | Primary and secondary aldehydes (alkanals) | Alkanes (saturated hydrocarbons) |
| Industrial Use | Production of aldehydes for plastics, detergents, and fragrances | Hydrogenation of oils, fats, and petrochemicals |
| Reaction Conditions | Pressure: 10-100 atm, Temp: 80-150degC | Pressure: 1-10 atm, Temp: 25-200degC |
| Mechanism | Insertion of alkene into metal-hydride bond followed by CO insertion and hydrogenolysis | Adsorption of H2 and unsaturated bond on catalyst surface followed by stepwise hydrogen addition |
| Applications | Intermediate synthesis in fine chemicals and pharmaceuticals | Food industry, petrochemical refining, synthetic fuel production |
Introduction to Hydroformylation and Hydrogenation
Hydroformylation is a chemical process that adds a formyl group and hydrogen to an alkene, producing aldehydes used in the synthesis of alcohols, plasticizers, and detergents. Hydrogenation involves the addition of hydrogen to unsaturated bonds in organic compounds, commonly converting alkenes to alkanes or reducing nitro compounds to amines. Your choice between hydroformylation and hydrogenation depends on whether the goal is to introduce functional groups like aldehydes or simply saturate double bonds for stability and reactivity changes.
Fundamental Principles of Hydroformylation
Hydroformylation, also known as the oxo process, involves the catalytic addition of a formyl group and hydrogen to an alkene, producing aldehydes. This reaction typically uses transition metal catalysts like rhodium or cobalt complexes under syngas (CO and H2) pressure. The fundamental principle hinges on the coordination of the alkene to the metal center, followed by insertion of CO and subsequent hydrogenation to form the aldehyde product.
Core Mechanism of Hydrogenation
Hydrogenation involves the addition of hydrogen (H2) across unsaturated bonds, typically using a metal catalyst such as palladium, platinum, or nickel to facilitate the reduction of alkenes or alkynes into alkanes. The core mechanism includes adsorption of both the alkene substrate and hydrogen molecules onto the catalyst surface, followed by successive transfer of hydrogen atoms to the carbon-carbon double bond, resulting in saturation. Understanding this mechanism helps optimize your reaction conditions for efficient conversion in industrial processes like fuel refining and chemical synthesis.
Catalysts Used in Hydroformylation vs Hydrogenation
Hydroformylation primarily utilizes cobalt or rhodium-based catalysts to facilitate the addition of a formyl group to alkenes, enhancing regioselectivity and efficiency. Hydrogenation generally relies on catalysts such as palladium, platinum, or nickel to add hydrogen across double bonds, effectively saturating unsaturated compounds. Understanding these catalysts helps you select the appropriate process based on reaction conditions and desired product outcomes.
Substrate Scope and Selectivity Differences
Hydroformylation primarily targets alkenes, converting them into aldehydes by adding a formyl group and hydrogen, showing high selectivity for terminal alkenes and allowing for regioselective control in product formation. Hydrogenation broadly reduces unsaturated compounds like alkenes, alkynes, and aromatic rings to saturated hydrocarbons with varying degrees of selectivity depending on catalyst choice and reaction conditions. Your selection between hydroformylation and hydrogenation should consider the desired functional group transformation and substrate compatibility to optimize yield and product specificity.
Industrial Applications: Hydroformylation vs Hydrogenation
Hydroformylation is widely used in the industrial production of aldehydes and alcohols, serving as a key step in manufacturing plasticizers, detergents, and intermediates for pharmaceuticals. Hydrogenation primarily focuses on converting unsaturated compounds into saturated derivatives, playing a critical role in producing edible fats, fuels, and active pharmaceutical ingredients. Both processes are essential in fine chemicals and petrochemical industries, with hydroformylation offering selective functionalization and hydrogenation enabling complete reduction of double bonds.
Environmental Impact and Sustainability Considerations
Hydroformylation and hydrogenation differ significantly in environmental impact and sustainability; hydroformylation often requires toxic catalysts like cobalt or rhodium and generates aldehyde byproducts that may pose disposal challenges. Hydrogenation typically uses less hazardous catalysts such as platinum or palladium, and its primary reaction product is saturated hydrocarbons, which are generally easier to handle and integrate into sustainable chemical cycles. Your choice between these processes should weigh catalyst toxicity, energy consumption, and waste generation to optimize environmental sustainability.
Process Efficiency and Reaction Conditions
Hydroformylation operates under moderate pressures (typically 10-100 atm) and temperatures between 80-150degC, achieving high selectivity for aldehyde production through the catalytic addition of syngas to alkenes. Hydrogenation usually requires higher pressures (20-200 atm) and temperatures ranging from 100-250degC, utilizing metal catalysts to add hydrogen across unsaturated bonds, enhancing reaction rates but sometimes reducing selectivity. Optimizing your process efficiency depends on balancing these reaction conditions to maximize yield while minimizing energy consumption and byproduct formation.
Advantages and Limitations of Each Process
Hydroformylation offers the advantage of converting alkenes into valuable aldehydes with high atom efficiency, enabling the synthesis of fine chemicals and intermediates for plastics and pharmaceuticals, but it requires expensive catalysts like rhodium or cobalt and operates under high pressure and temperature. Hydrogenation efficiently saturates unsaturated bonds to produce alkanes, widely used in food processing and petrochemical refining, showcasing high selectivity and lower catalyst costs, though it often demands harsh reaction conditions and can lack functional group tolerance. Both processes are integral to industrial chemistry, yet hydroformylation's complexity and catalyst sensitivity contrast with the broader applicability and operational simplicity of hydrogenation.
Future Trends and Innovations in Hydroformylation and Hydrogenation
Future trends in hydroformylation emphasize the development of more selective and sustainable catalysts, including transition metal complexes and biocatalysts, which enhance efficiency and reduce environmental impact. Hydrogenation is advancing through innovations such as continuous flow reactors and electrocatalytic methods, improving reaction control and energy efficiency. Your applications will benefit from these innovations by achieving higher precision and greener processes in chemical synthesis.
Hydroformylation vs hydrogenation Infographic
 libmatt.com
libmatt.com