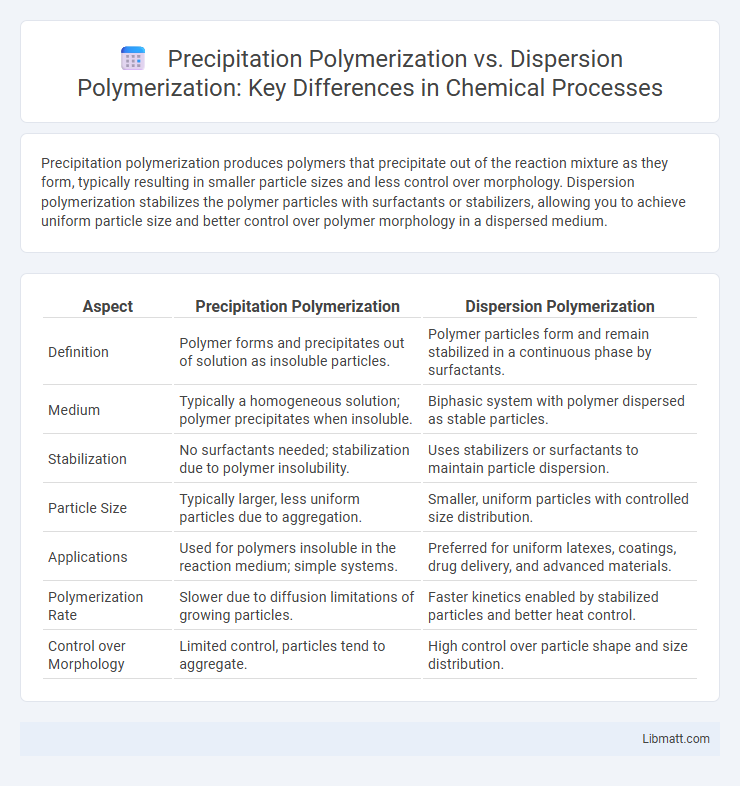
Precipitation polymerization produces polymers that precipitate out of the reaction mixture as they form, typically resulting in smaller particle sizes and less control over morphology. Dispersion polymerization stabilizes the polymer particles with surfactants or stabilizers, allowing you to achieve uniform particle size and better control over polymer morphology in a dispersed medium.
Table of Comparison
| Aspect | Precipitation Polymerization | Dispersion Polymerization |
|---|---|---|
| Definition | Polymer forms and precipitates out of solution as insoluble particles. | Polymer particles form and remain stabilized in a continuous phase by surfactants. |
| Medium | Typically a homogeneous solution; polymer precipitates when insoluble. | Biphasic system with polymer dispersed as stable particles. |
| Stabilization | No surfactants needed; stabilization due to polymer insolubility. | Uses stabilizers or surfactants to maintain particle dispersion. |
| Particle Size | Typically larger, less uniform particles due to aggregation. | Smaller, uniform particles with controlled size distribution. |
| Applications | Used for polymers insoluble in the reaction medium; simple systems. | Preferred for uniform latexes, coatings, drug delivery, and advanced materials. |
| Polymerization Rate | Slower due to diffusion limitations of growing particles. | Faster kinetics enabled by stabilized particles and better heat control. |
| Control over Morphology | Limited control, particles tend to aggregate. | High control over particle shape and size distribution. |
Introduction to Precipitation and Dispersion Polymerization
Precipitation polymerization involves the polymer forming as insoluble particles that precipitate out of the reaction medium, typically a homogeneous solution, once the polymer reaches a critical molecular weight. Dispersion polymerization occurs when the polymer forms stable colloidal particles within the reaction medium, often stabilized by surfactants or stabilizers to prevent aggregation. Both methods enable the synthesis of polymer particles with distinct morphologies, sizes, and applications in coatings, drug delivery, and materials science.
Overview of Polymerization Techniques
Precipitation polymerization involves the formation of insoluble polymer particles that precipitate out of the solution as the reaction proceeds, typically used for producing uniform microspheres with controlled size. Dispersion polymerization also generates polymer particles in a continuous phase but employs stabilizers to prevent aggregation, resulting in stable colloidal dispersions ideal for producing monodisperse latex particles. Both techniques operate in heterogeneous media but differ primarily in particle stabilization and polymer solubility, influencing particle size distribution and application suitability.
Fundamental Principles of Precipitation Polymerization
Precipitation polymerization involves the polymerization of monomers in a solvent where the formed polymer is insoluble, causing it to precipitate out as particles. This method relies on the polymer's solubility difference between the monomer phase and the polymer phase, leading to nucleation and growth of polymer particles without stabilizers. The insolubility of the polymer in the reaction medium ensures particle formation, distinguishing it from dispersion polymerization where stabilizers and particle stabilization mechanisms are critical.
Core Mechanisms in Dispersion Polymerization
Dispersion polymerization involves stabilizing polymer particles formed in a continuous phase, typically a solvent in which monomers are soluble but polymers are not, leading to nucleation and growth of polymer beads stabilized by surfactants or polymeric stabilizers. Unlike precipitation polymerization, which relies on polymer insolubility for particle formation without stabilizers, dispersion polymerization ensures uniform particle size distribution through steric stabilization. Your choice between these methods hinges on desired particle morphology, size control, and reaction medium compatibility.
Key Differences Between Precipitation and Dispersion Polymerization
Precipitation polymerization involves polymer formation in a solvent where polymers precipitate out as they grow, leading to heterogeneous particle formation typically without surfactants, while dispersion polymerization uses stabilizers or surfactants to maintain polymer particles dispersed in the continuous phase. The particle size in precipitation polymerization tends to be larger and less uniform compared to the controlled, smaller, and more uniform particles obtained in dispersion polymerization. Reaction kinetics differ as well, with precipitation polymerization often exhibiting faster polymerization rates due to immediate polymer precipitation, whereas dispersion polymerization allows for better control over molecular weight and particle morphology.
Particle Formation and Morphology Control
Precipitation polymerization typically results in the formation of particles through nucleation followed by growth, producing relatively larger and more polydisperse particles with less control over morphology. Dispersion polymerization, by contrast, offers superior morphology control due to the use of stabilizers that prevent particle agglomeration, enabling the production of monodisperse, uniform spherical particles. Particle formation in dispersion polymerization occurs via controlled nucleation and growth within a continuous phase, resulting in well-defined particle sizes and shapes.
Monomer and Solvent Selection Criteria
In precipitation polymerization, monomers must be soluble in the solvent while the resulting polymer is insoluble, causing polymer particles to precipitate out; solvents are selected based on their ability to dissolve monomers but not polymers, such as water or alcohols for hydrophobic monomers. Dispersion polymerization requires both monomers and initiators to be soluble in the continuous phase, while the polymer remains insoluble, enabling particle nucleation and growth; solvents are typically polar or nonpolar organic media adjusted to stabilize colloidal particles with appropriate stabilizers. Selecting monomers involves balancing solubility and polymer insolubility, whereas solvent choice hinges on controlling particle size, stability, and polymerization kinetics in both methods.
Advantages and Limitations of Each Method
Precipitation polymerization offers advantages such as producing uniform polymer microspheres with narrow size distribution and simple separation without requiring stabilizers, but it is limited by low monomer concentrations and potential particle aggregation. Dispersion polymerization provides better control over particle size and morphology with higher monomer loadings, using stabilizers to maintain stability, yet it can suffer from residual stabilizer contamination affecting polymer purity. Each method's suitability depends on desired particle size, polymer properties, and process scalability in applications such as coatings, drug delivery, and composite materials.
Major Industrial and Research Applications
Precipitation polymerization is widely used in the production of uniform polymer microspheres for biomedical applications, drug delivery systems, and chromatography media due to its ability to produce particles with narrow size distribution. Dispersion polymerization finds major industrial use in coatings, adhesives, and latex production, offering better control over particle size and stability in non-aqueous solvents. Understanding the differences in particle formation mechanisms helps optimize Your polymer design for specific industrial and research applications, improving efficiency and product performance.
Future Perspectives and Emerging Trends
Future perspectives in precipitation polymerization include the development of environmentally friendly solvents and precise control over particle size for advanced drug delivery systems. Emerging trends in dispersion polymerization emphasize sustainable monomers and smart polymers responsive to external stimuli, enhancing applications in coatings and biomedical fields. Your ability to tailor polymer structures through these evolving techniques will expand opportunities in nanotechnology and materials science.
Precipitation polymerization vs dispersion polymerization Infographic
 libmatt.com
libmatt.com