Titration measures the concentration of a solution through a controlled reaction with a standard reagent, providing rapid and precise results ideal for routine analysis. Gravimetric analysis determines the amount of an analyte by isolating and weighing a solid product, offering high accuracy for quantifying substances in complex mixtures.
Table of Comparison
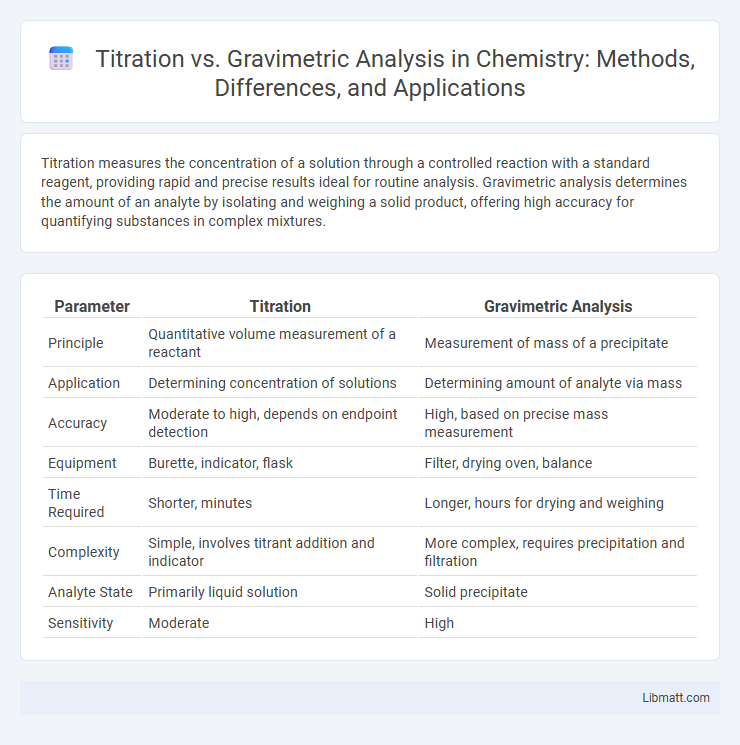
| Parameter | Titration | Gravimetric Analysis |
|---|---|---|
| Principle | Quantitative volume measurement of a reactant | Measurement of mass of a precipitate |
| Application | Determining concentration of solutions | Determining amount of analyte via mass |
| Accuracy | Moderate to high, depends on endpoint detection | High, based on precise mass measurement |
| Equipment | Burette, indicator, flask | Filter, drying oven, balance |
| Time Required | Shorter, minutes | Longer, hours for drying and weighing |
| Complexity | Simple, involves titrant addition and indicator | More complex, requires precipitation and filtration |
| Analyte State | Primarily liquid solution | Solid precipitate |
| Sensitivity | Moderate | High |
Introduction to Titration and Gravimetric Analysis
Titration is a quantitative analytical technique that determines the concentration of an unknown solution by reacting it with a standard solution of known concentration, often using indicators to identify the endpoint. Gravimetric analysis measures the mass of an analyte or its derivative after precipitation to calculate its concentration with high precision. Understanding these methods helps you select the appropriate approach based on the required accuracy and sample characteristics.
Principles of Titration
Titration is an analytical technique based on the precise measurement of the volume of a titrant required to react completely with an analyte, following a stoichiometric chemical reaction. The endpoint is determined using indicators or potentiometric methods, ensuring accurate quantification of the analyte concentration. This contrasts with gravimetric analysis, which relies on measuring mass changes rather than volume.
Fundamentals of Gravimetric Analysis
Gravimetric analysis relies on the precise measurement of mass to determine the concentration of an analyte by converting it into a stable, insoluble compound that can be filtered, dried, and weighed. This technique emphasizes the careful precipitation and isolation of the compound to achieve high accuracy and reproducibility in quantitative chemical analysis. Unlike titration, gravimetric analysis provides direct measurement through mass determination, minimizing errors associated with volumetric methods.
Key Differences Between Titration and Gravimetric Analysis
Titration involves measuring the volume of a solution of known concentration required to react completely with an analyte, providing rapid and precise quantitative results for liquids. Gravimetric analysis relies on the measurement of mass, where the analyte is converted into a solid precipitate, offering high accuracy and purity but typically requiring more time. You should choose titration for faster analysis and gravimetric methods when precise mass determination and purity assessment are essential.
Types of Titration Methods
Titration methods include acid-base titration, redox titration, complexometric titration, and precipitation titration, each designed for specific types of chemical reactions and analytes. Acid-base titration measures pH changes to determine concentrations, while redox titration relies on oxidation-reduction reactions. Complexometric titrations use chelating agents to quantify metal ions, and precipitation titrations form insoluble compounds to enable precise analysis, contrasting with gravimetric analysis, which measures mass changes directly.
Types of Gravimetric Analysis Techniques
Gravimetric analysis techniques include precipitation gravimetry, where an analyte is converted into an insoluble solid for filtration and weighing, and volatilization gravimetry, which involves measuring the mass change after vaporizing a component. Other methods involve complexometric gravimetry, forming complexes that precipitate for quantification, and electrogravimetry, where the analyte deposits on an electrode surface. These techniques provide precise quantitative results by relying on mass measurements, contrasting with titration's volumetric approach.
Accuracy and Precision: Comparing Both Methods
Titration offers high precision due to the controlled measurement of reagent volume, making it suitable for determining concentrations with minimal error. Gravimetric analysis provides superior accuracy by measuring mass changes directly, reducing the influence of instrument calibration and operator skill. When selecting a method, consider that titration yields rapid results with good reproducibility, while gravimetric analysis delivers more exact quantitative data, especially for pure substances.
Advantages and Limitations of Titration
Titration offers rapid and precise quantitative analysis, making it ideal for determining the concentration of an analyte with minimal sample preparation. It provides real-time results and is cost-effective, requiring relatively simple equipment and reagents. However, titration depends on the availability of suitable indicators and can be less accurate for colored or turbid solutions, with limitations in detecting low analyte concentrations compared to gravimetric analysis.
Pros and Cons of Gravimetric Analysis
Gravimetric analysis offers high accuracy and reliability by directly measuring the mass of an analyte, making it ideal for pure substance quantification without the need for complex equipment. However, it can be time-consuming due to multiple steps such as precipitation, filtration, and drying, and may require careful control of experimental conditions to avoid losses or contamination. Your choice between gravimetric and titration methods depends on the required precision, sample nature, and available laboratory resources.
Applications in Real-world Chemical Analysis
Titration is widely used for determining the concentration of acids, bases, and redox-active compounds in pharmaceuticals, water quality testing, and food chemistry due to its rapid and precise endpoint detection. Gravimetric analysis excels in measuring the purity of metal ores, determining sulfate content in minerals, and quantifying precipitates in environmental samples through highly accurate mass measurement. Both techniques are essential in industrial quality control and environmental monitoring, offering complementary strengths in quantitative chemical analysis.
titration vs gravimetric analysis Infographic
 libmatt.com
libmatt.com