Pyrolysis and gasification are thermal processes that convert organic materials into valuable energy products, but pyrolysis decomposes feedstock in the absence of oxygen producing mainly bio-oil, char, and syngas, while gasification partially oxidizes materials at high temperatures generating primarily syngas composed of hydrogen, carbon monoxide, and methane. Understanding the differences between these technologies can help you select the optimal method for waste-to-energy conversion or renewable fuel production.
Table of Comparison
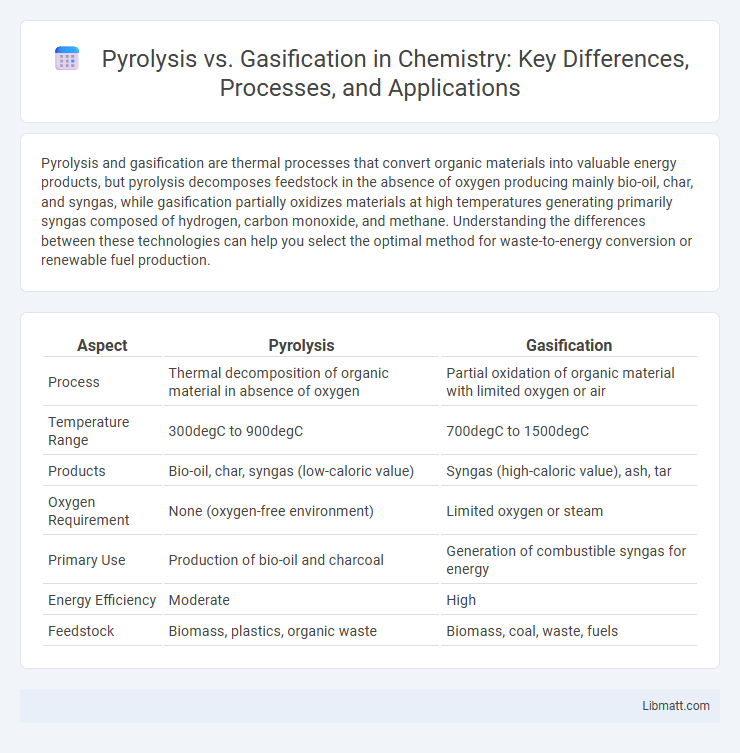
| Aspect | Pyrolysis | Gasification |
|---|---|---|
| Process | Thermal decomposition of organic material in absence of oxygen | Partial oxidation of organic material with limited oxygen or air |
| Temperature Range | 300degC to 900degC | 700degC to 1500degC |
| Products | Bio-oil, char, syngas (low-caloric value) | Syngas (high-caloric value), ash, tar |
| Oxygen Requirement | None (oxygen-free environment) | Limited oxygen or steam |
| Primary Use | Production of bio-oil and charcoal | Generation of combustible syngas for energy |
| Energy Efficiency | Moderate | High |
| Feedstock | Biomass, plastics, organic waste | Biomass, coal, waste, fuels |
Introduction to Pyrolysis and Gasification
Pyrolysis and gasification are advanced thermal processes used to convert organic materials into valuable energy products. Pyrolysis involves heating biomass in the absence of oxygen, producing bio-oil, syngas, and char, while gasification partially oxidizes feedstock to generate syngas rich in hydrogen and carbon monoxide. Understanding these processes helps optimize your choice for sustainable energy production and waste management solutions.
Defining Pyrolysis: Principles and Processes
Pyrolysis is a thermochemical decomposition process occurring in an oxygen-limited environment, breaking down organic materials at temperatures typically between 400degC and 800degC. This process produces valuable products such as biochar, bio-oil, and syngas by cracking complex polymers into simpler molecules without combustion. The principles of pyrolysis involve thermal degradation, volatilization, and condensation, making it distinct from gasification, which operates with controlled oxygen to generate primarily syngas.
Understanding Gasification: Key Concepts
Gasification is a thermochemical process that converts organic materials into syngas, a mixture of carbon monoxide, hydrogen, and methane, through partial oxidation at high temperatures. Unlike pyrolysis, which occurs in the absence of oxygen, gasification utilizes a controlled amount of oxygen or steam to enable chemical reactions that produce combustible gases. Understanding gasification helps optimize energy recovery from biomass and waste, enhancing efficiency and reducing environmental impact for Your renewable energy projects.
Feedstock Requirements and Variations
Pyrolysis and gasification differ significantly in feedstock requirements and variations; pyrolysis can process a wide range of organic materials including biomass, plastics, and waste with varying moisture content up to 30%, while gasification typically requires drier feedstock with moisture below 20% for efficient syngas production. Pyrolysis operates effectively with heterogeneous feedstocks, producing bio-oil, char, and syngas, whereas gasification demands more uniform and finely sized feedstock to maintain high-temperature oxygen-limited conditions essential for converting carbonaceous materials into combustible gases. The flexibility in feedstock type makes pyrolysis advantageous for diverse waste streams, contrasting with gasification's preference for controlled biomass or coal feeds to optimize energy output and minimize tar formation.
Operational Temperature and Atmospheric Conditions
Pyrolysis operates at lower temperatures, typically between 300degC and 700degC, under an inert atmosphere to thermally decompose organic materials without combustion. Gasification occurs at higher temperatures, usually ranging from 800degC to 1,200degC, in a controlled environment with limited oxygen or steam to partially oxidize the feedstock and produce syngas. The atmospheric conditions in pyrolysis prevent oxidation, while gasification's partial oxidation is critical for sustaining the endothermic reactions and syngas generation.
Main Products: Outputs of Each Technology
Pyrolysis primarily produces bio-oil, syngas, and biochar by thermally decomposing organic materials in the absence of oxygen. Gasification converts biomass or coal into syngas--a mixture of hydrogen, carbon monoxide, and carbon dioxide--by partial oxidation at high temperatures. Your choice between pyrolysis and gasification depends on the desired output, with pyrolysis favoring liquid fuels and char, while gasification excels in generating a combustible gas for energy or chemical synthesis.
Energy Efficiency and Conversion Rates
Pyrolysis typically achieves higher energy efficiency by converting organic materials into bio-oil, syngas, and char through thermal decomposition in the absence of oxygen, with energy conversion rates often ranging between 60-75%. Gasification operates at higher temperatures with limited oxygen, producing mainly syngas with energy conversion rates around 70-85%, enabling more efficient fuel synthesis and power generation. Your choice between pyrolysis and gasification depends on the desired output and process efficiency requirements for specific feedstock utilization.
Environmental Impacts and Emissions Comparison
Pyrolysis produces lower emissions of nitrogen oxides (NOx) and sulfur oxides (SOx) compared to gasification, making it a cleaner option for reducing air pollution. Gasification generates more syngas with higher hydrogen content but can release higher levels of particulate matter and tar, which require advanced cleanup systems to minimize environmental impact. Your choice between pyrolysis and gasification should consider the balance between energy output efficiency and the need for emissions control technologies to protect air quality.
Industrial Applications and Use Cases
Pyrolysis is widely used in industrial applications for converting plastic waste and biomass into bio-oil, syngas, and char, serving sectors like renewable energy production and chemical manufacturing. Gasification finds extensive use in producing synthetic fuels, electricity, and hydrogen from coal, biomass, or municipal solid waste, supporting industries aiming for cleaner energy and waste reduction. Your choice between these technologies depends on specific feedstock availability, desired output composition, and environmental regulations shaping sustainable industrial processes.
Future Trends and Innovations in Thermochemical Conversion
Future trends in thermochemical conversion emphasize enhancing the efficiency and sustainability of pyrolysis and gasification processes through advancements in catalyst development, reactor design, and feedstock diversification. Innovations include integrating AI-driven process control systems and hybrid technologies that combine pyrolysis with gasification to maximize energy recovery and minimize emissions. Research also focuses on scaling modular systems and utilizing bio-waste, aiming to develop carbon-neutral energy solutions aligned with circular economy principles.
Pyrolysis vs gasification Infographic
 libmatt.com
libmatt.com