Arrhenius acids increase hydrogen ion (H+) concentration in aqueous solutions, while Lewis acids accept an electron pair during chemical reactions. Understanding this distinction helps you identify acids based on their behavior in different chemical environments.
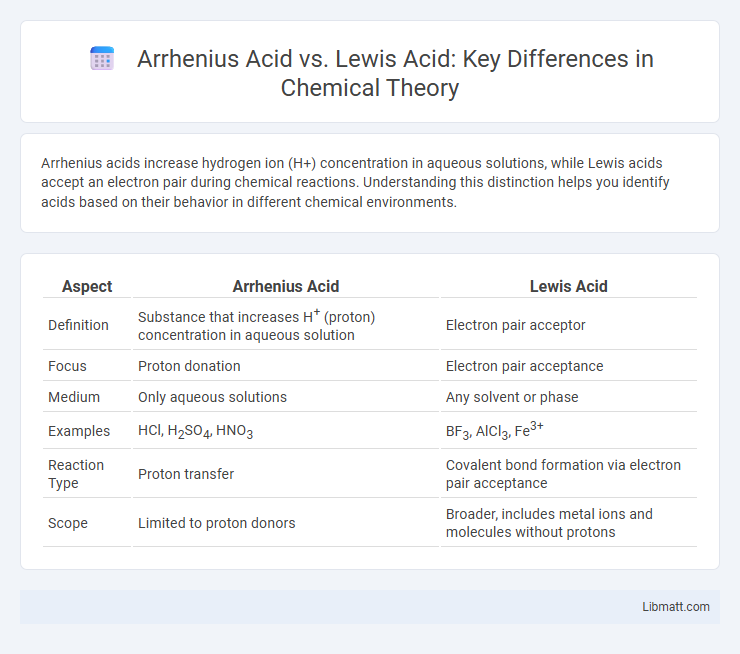
Table of Comparison
| Aspect | Arrhenius Acid | Lewis Acid |
|---|---|---|
| Definition | Substance that increases H+ (proton) concentration in aqueous solution | Electron pair acceptor |
| Focus | Proton donation | Electron pair acceptance |
| Medium | Only aqueous solutions | Any solvent or phase |
| Examples | HCl, H2SO4, HNO3 | BF3, AlCl3, Fe3+ |
| Reaction Type | Proton transfer | Covalent bond formation via electron pair acceptance |
| Scope | Limited to proton donors | Broader, includes metal ions and molecules without protons |
Introduction to Acid Theories
Arrhenius acids are substances that increase the concentration of H+ ions in an aqueous solution, while Lewis acids are defined by their ability to accept an electron pair from a Lewis base. Arrhenius theory is limited to aqueous solutions and focuses on proton donation, whereas Lewis theory expands the concept of acidity beyond protons to include electron pair acceptance, broadening the range of acid-base reactions. Understanding the distinction between Arrhenius and Lewis acids is essential for predicting reactivity in various chemical environments and applications.
Defining Arrhenius Acids
Arrhenius acids are substances that increase the concentration of hydrogen ions (H+) in an aqueous solution, exemplified by HCl dissociating into H+ and Cl- in water. This definition is limited to aqueous environments and proton donation. Lewis acids, in contrast, are electron pair acceptors and are not restricted to protons or water-based chemistry.
Defining Lewis Acids
Lewis acids are chemical species that can accept an electron pair from a Lewis base to form a coordinate covalent bond. Unlike Arrhenius acids, which increase hydrogen ion concentration in aqueous solutions, Lewis acids are defined by their ability to accept electron pairs regardless of the presence of protons. Common examples of Lewis acids include metal ions like Fe3+ and molecules such as BF3 and AlCl3, which have vacant orbitals to accept electron pairs during reactions.
Key Differences Between Arrhenius and Lewis Acids
Arrhenius acids increase the concentration of hydrogen ions (H+) in aqueous solutions, whereas Lewis acids act as electron pair acceptors in chemical reactions regardless of the solvent. Arrhenius definition is limited to aqueous environments, while Lewis acids encompass a broader range of substances including metal cations and molecules with vacant orbitals. This fundamental difference highlights Arrhenius acids' role in proton donation contrasted with Lewis acids' involvement in electron pair interactions.
Molecular Examples of Arrhenius Acids
Hydrochloric acid (HCl) and sulfuric acid (H2SO4) serve as classic molecular examples of Arrhenius acids, releasing H+ ions in aqueous solutions. Acetic acid (CH3COOH) also exemplifies an Arrhenius acid by partially dissociating to yield protons in water. These substances contrast with Lewis acids, which accept electron pairs without necessarily releasing protons.
Molecular Examples of Lewis Acids
Lewis acids are molecules or ions that accept an electron pair during chemical reactions, with common examples including boron trifluoride (BF3), aluminum chloride (AlCl3), and iron(III) chloride (FeCl3). These compounds have empty orbitals or electron-deficient centers that facilitate the acceptance of electron pairs from Lewis bases. Understanding your interaction with Lewis acids can help predict reaction mechanisms and design catalytic processes in organic and inorganic chemistry.
Scope and Limitations of Each Acid Theory
Arrhenius acid theory is limited to aqueous solutions and defines acids strictly as proton (H+) donors, restricting its scope to substances that increase hydrogen ion concentration in water. Lewis acid theory has a broader scope, encompassing any electron pair acceptor regardless of solvent or the presence of protons, which allows it to explain acid-base behavior in non-aqueous and complex chemical systems. Your understanding of acid-base reactions expands significantly by recognizing these limitations and applying the appropriate theory based on the chemical environment.
Real-World Applications of Arrhenius and Lewis Acids
Arrhenius acids, like hydrochloric acid in stomach digestion and sulfuric acid in battery production, are crucial for processes involving proton donation and pH regulation. Lewis acids, such as aluminum chloride used in catalytic converters and boron trifluoride in organic synthesis, play key roles in industrial catalysis and chemical manufacturing by accepting electron pairs. Understanding the distinct reactivity of Arrhenius and Lewis acids helps you optimize chemical reactions for pharmaceuticals, environmental technologies, and materials science.
Acid Strength: Arrhenius vs Lewis Perspective
Arrhenius acids increase hydrogen ion concentration in aqueous solutions, with strength defined by the extent of dissociation into H+ ions, making concentration and ionization key factors. Lewis acids accept electron pairs, so their strength depends on the ability to accept electrons from Lewis bases, influenced by factors like electrophilicity and vacant orbitals. Understanding Your application context helps determine which acid strength perspective--Arrhenius for proton donation or Lewis for electron pair acceptance--is more relevant.
Summary and Comparative Analysis
Arrhenius acids release hydrogen ions (H+) in aqueous solutions, while Lewis acids accept electron pairs from donor species, broadening their scope beyond aqueous environments. Your understanding benefits from recognizing that Arrhenius acids are limited to proton donors, whereas Lewis acids encompass a wider range of electron pair acceptors, including metal ions and molecules without hydrogen. This fundamental difference highlights Lewis acids' versatility in chemical reactions compared to the more specific definition of Arrhenius acids.
Arrhenius acid vs Lewis acid Infographic
 libmatt.com
libmatt.com