An ammonia plant synthesizes ammonia primarily through the Haber-Bosch process by combining nitrogen and hydrogen, whereas a methanol plant produces methanol by catalytically converting synthesis gas, a mixture of carbon monoxide, carbon dioxide, and hydrogen. Understanding the distinct feedstock requirements and product applications helps optimize your choice for industrial chemistry or fuel production.
Table of Comparison
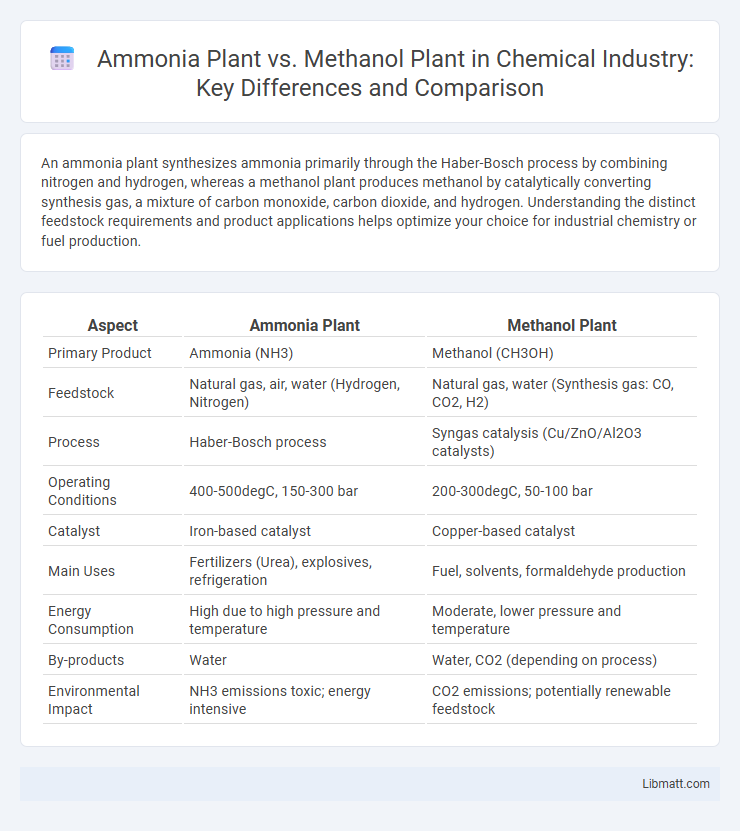
| Aspect | Ammonia Plant | Methanol Plant |
|---|---|---|
| Primary Product | Ammonia (NH3) | Methanol (CH3OH) |
| Feedstock | Natural gas, air, water (Hydrogen, Nitrogen) | Natural gas, water (Synthesis gas: CO, CO2, H2) |
| Process | Haber-Bosch process | Syngas catalysis (Cu/ZnO/Al2O3 catalysts) |
| Operating Conditions | 400-500degC, 150-300 bar | 200-300degC, 50-100 bar |
| Catalyst | Iron-based catalyst | Copper-based catalyst |
| Main Uses | Fertilizers (Urea), explosives, refrigeration | Fuel, solvents, formaldehyde production |
| Energy Consumption | High due to high pressure and temperature | Moderate, lower pressure and temperature |
| By-products | Water | Water, CO2 (depending on process) |
| Environmental Impact | NH3 emissions toxic; energy intensive | CO2 emissions; potentially renewable feedstock |
Overview of Ammonia and Methanol Plants
Ammonia plants primarily produce ammonia by synthesizing nitrogen and hydrogen through the Haber-Bosch process, which is essential for fertilizers and industrial chemicals. Methanol plants convert synthesis gas, a mixture of carbon monoxide, carbon dioxide, and hydrogen, into methanol used as a fuel, solvent, and chemical feedstock. Your choice between an ammonia plant and a methanol plant depends on market demand, raw material availability, and end-use applications in agriculture or energy sectors.
Key Differences in Production Processes
Ammonia plants primarily utilize the Haber-Bosch process, which synthesizes ammonia by reacting nitrogen from the air with hydrogen typically derived from natural gas through steam methane reforming. Methanol plants, on the other hand, produce methanol by catalytic synthesis of carbon monoxide and hydrogen, where syngas is obtained via similar steam methane reforming but processed differently to optimize the H2:CO ratio. The key differences lie in the feedstock composition and catalytic pathways, with ammonia production focusing on nitrogen fixation and methanol production emphasizing carbon monoxide conversion.
Raw Materials and Feedstock Comparison
Ammonia plants primarily utilize natural gas or other hydrocarbons as feedstock, which undergoes reforming to produce hydrogen and nitrogen from air for synthesis. Methanol plants also rely on natural gas but convert it into synthesis gas, a mixture of hydrogen, carbon monoxide, and carbon dioxide, as the main raw material for methanol production. Understanding the feedstock differences, your choice between ammonia and methanol plants hinges on the desired end product and the availability of suitable hydrocarbons and synthesis gas components.
Major Industrial Applications
Ammonia plants primarily serve the fertilizer industry, producing ammonia essential for nitrogen-based fertilizers, as well as playing a key role in the manufacture of explosives and cleaning products. Methanol plants supply a versatile feedstock for producing formaldehyde, acetic acid, and various solvents, with significant applications in fuel production and chemical synthesis. Your choice between ammonia and methanol plants depends on whether your industrial focus is agriculture and cleaning products or chemical manufacturing and alternative fuels.
Environmental Impact and Emissions
Ammonia plants primarily emit nitrogen oxides (NOx), carbon dioxide (CO2), and unreacted ammonia, contributing significantly to greenhouse gas emissions and potential atmospheric pollution. Methanol plants generate CO2 and volatile organic compounds (VOCs), with emissions heavily dependent on feedstock and production processes, often resulting in lower NOx levels compared to ammonia production. Advanced technologies in both plants aim to reduce carbon footprints through carbon capture and process optimization, yet ammonia synthesis remains more energy-intensive and associated with higher environmental impacts.
Plant Design and Engineering Features
Ammonia plants utilize the Haber-Bosch process, requiring high-pressure reactors and synthesis loops operating typically at 150-300 bar and 400-500degC, emphasizing robust nitrogen and hydrogen separation units. Methanol plants implement low-pressure catalytic reactors at around 50-100 bar and 200-300degC, integrating advanced syngas purification and energy recovery systems to maximize efficiency. Both plants feature sophisticated control systems, but ammonia plants generally demand more intensive catalyst management and corrosion-resistant materials due to harsher operating conditions.
Energy Consumption and Efficiency
Ammonia plants typically consume around 28-35 GJ of energy per tonne of ammonia produced, relying heavily on natural gas feedstock and steam methane reforming, which affects overall efficiency. Methanol plants, on the other hand, generally have an energy consumption in the range of 32-38 GJ per tonne, with efficiency influenced by the choice of synthesis gas feed and process configurations such as CO2 utilization. Advanced process optimizations and heat integration in both ammonia and methanol plants are critical for reducing fuel consumption and improving thermal efficiency, often achieving energy efficiency rates above 60%.
Safety Protocols and Hazard Management
Ammonia plants require stringent safety protocols due to the toxicity and high-pressure conditions of ammonia production, emphasizing continuous monitoring for leaks and effective ventilation systems. Methanol plants focus heavily on managing flammability risks and controlling formaldehyde exposure, implementing advanced fire suppression and explosion-proof equipment. Your facility's hazard management should tailor safety measures to these specific chemical properties to ensure operational safety and regulatory compliance.
Economic Viability and Market Demand
Ammonia plants and methanol plants differ significantly in economic viability due to feedstock costs, capacity scales, and product pricing stability, with ammonia benefiting from steady demand in fertilizer production. Methanol plants respond dynamically to market fluctuations, driven by its diverse applications in fuel, chemicals, and energy sectors, impacting profitability and investment attractiveness. Evaluating your project requires analyzing regional natural gas prices, market demand projections for fertilizers versus chemical intermediates, and potential revenue streams to determine the optimal plant type.
Future Trends in Ammonia and Methanol Production
Ammonia plants are increasingly adopting green hydrogen produced via electrolysis powered by renewable energy, aligning with global decarbonization goals and reducing carbon emissions in fertilizer manufacturing. Methanol plants are shifting towards bio-methanol and e-methanol production, utilizing biomass feedstocks and renewable hydrogen, which supports circular economy principles and lowers dependence on fossil fuels. Both industries are investing in advanced catalyst technologies and carbon capture integration to enhance efficiency and sustainability in future production processes.
Ammonia plant vs methanol plant Infographic
 libmatt.com
libmatt.com