Isoelectric precipitation involves the aggregation of proteins at their isoelectric point, where they have no net charge and thus become insoluble, while heat coagulation causes protein denaturation and aggregation through the application of high temperatures. Your choice between these methods depends on the specific protein properties and desired purity or texture in food and biochemical processing.
Table of Comparison
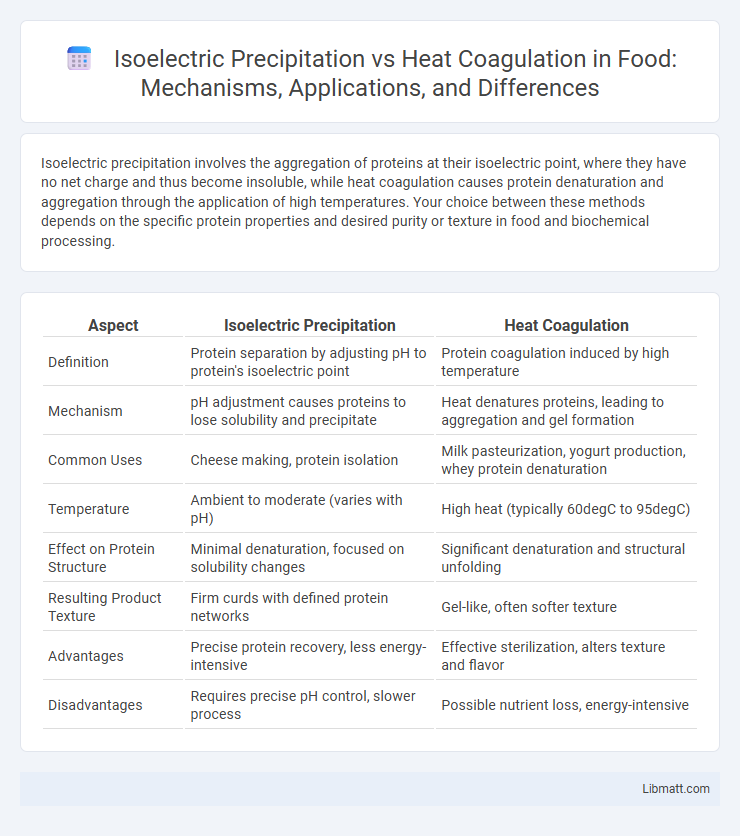
| Aspect | Isoelectric Precipitation | Heat Coagulation |
|---|---|---|
| Definition | Protein separation by adjusting pH to protein's isoelectric point | Protein coagulation induced by high temperature |
| Mechanism | pH adjustment causes proteins to lose solubility and precipitate | Heat denatures proteins, leading to aggregation and gel formation |
| Common Uses | Cheese making, protein isolation | Milk pasteurization, yogurt production, whey protein denaturation |
| Temperature | Ambient to moderate (varies with pH) | High heat (typically 60degC to 95degC) |
| Effect on Protein Structure | Minimal denaturation, focused on solubility changes | Significant denaturation and structural unfolding |
| Resulting Product Texture | Firm curds with defined protein networks | Gel-like, often softer texture |
| Advantages | Precise protein recovery, less energy-intensive | Effective sterilization, alters texture and flavor |
| Disadvantages | Requires precise pH control, slower process | Possible nutrient loss, energy-intensive |
Introduction to Protein Precipitation Methods
Protein precipitation methods are essential for isolating and purifying proteins based on their solubility changes under different conditions. Isoelectric precipitation leverages the protein's isoelectric point, where its net charge is zero, causing it to aggregate and precipitate out of solution. Heat coagulation relies on elevated temperatures to denature proteins, resulting in aggregation and precipitation, a process crucial for understanding protein stability and function.
Overview of Isoelectric Precipitation
Isoelectric precipitation is a technique used to separate proteins by adjusting the pH to their isoelectric point, where their net charge is zero, leading to minimal solubility and aggregation. This method is widely applied in dairy processing and protein isolation to achieve high purity without thermal denaturation. Unlike heat coagulation, which uses elevated temperatures to unfold and aggregate proteins, isoelectric precipitation preserves native protein structures and functional properties.
Overview of Heat Coagulation
Heat coagulation is a process where proteins denature and aggregate when exposed to elevated temperatures, commonly used in dairy and food industries to induce gel formation or curdling. This technique relies on heat-induced unfolding of protein structures, leading to irreversible aggregation and precipitation, which differs from isoelectric precipitation that depends on pH adjustments to reach the protein's isoelectric point. Heat coagulation is critical in applications such as cheese making, yogurt production, and thermal processing of milk-based products to achieve desired texture and stability.
Principle and Mechanism: Isoelectric Precipitation
Isoelectric precipitation separates proteins by adjusting the pH to their isoelectric point, where they have no net electrical charge and thus aggregate and precipitate out of solution. This process relies on reducing electrostatic repulsion, allowing proteins to clump and form solid particles that can be easily separated. Your understanding of this principle helps in optimizing protein recovery and purity in various applications, such as food processing and biopharmaceutical production.
Principle and Mechanism: Heat Coagulation
Heat coagulation involves denaturing proteins by applying high temperatures, causing their three-dimensional structures to unfold and expose hydrophobic groups that aggregate irreversibly. The process disrupts non-covalent bonds such as hydrogen bonds and hydrophobic interactions, leading to protein coagulum formation through increased particle interactions and network development. This thermal denaturation differs from isoelectric precipitation, which relies on pH adjustment to the protein's isoelectric point to reduce solubility and induce coagulation.
Factors Affecting Isoelectric Precipitation
Isoelectric precipitation is primarily influenced by pH, temperature, ionic strength, and protein concentration, with the optimal pH at the protein's isoelectric point causing minimal solubility and maximum precipitation. In contrast, heat coagulation depends largely on temperature, heating time, protein denaturation properties, and the presence of minerals like calcium, which destabilize protein structures and promote aggregation. Understanding the modulation of these factors is essential for controlling texture and yield in dairy processing and protein isolation.
Factors Influencing Heat Coagulation
Heat coagulation in dairy processing is primarily influenced by factors such as pH, protein concentration, and the presence of salts like calcium. Temperature and heating duration significantly affect protein denaturation and aggregation, with higher heat and longer times promoting coagulation. Your control over these variables ensures optimal product texture and stability during processing.
Key Differences: Isoelectric Precipitation vs Heat Coagulation
Isoelectric precipitation involves adjusting the pH to the protein's isoelectric point, causing proteins to lose solubility and precipitate out of solution with minimal heat exposure. Heat coagulation, by contrast, relies on thermal denaturation, where elevated temperatures disrupt protein structure leading to aggregation and gel formation. The key differences lie in the mechanism--pH-driven solubility change versus temperature-induced denaturation--and the resulting protein structure and functional properties.
Applications in Food and Dairy Industries
Isoelectric precipitation is widely used in cheese manufacturing to separate casein proteins by adjusting pH to their isoelectric point, enhancing curd formation and cheese yield. Heat coagulation is crucial in producing heat-stable dairy products like yogurt and evaporated milk, where controlled denaturation of whey proteins improves texture and shelf life. Both methods optimize protein functionality for diverse applications in food and dairy industries, including gelatin production, tofu making, and protein isolation.
Conclusion: Choosing the Right Protein Separation Technique
Isoelectric precipitation offers precise protein separation by exploiting pH-based solubility changes, making it ideal for purifying proteins with specific isoelectric points. Heat coagulation relies on temperature-induced denaturation but may cause irreversible aggregation, limiting its use for sensitive proteins. Selecting the appropriate method depends on the protein's stability and desired purity, with isoelectric precipitation preferred for specificity and heat coagulation suitable for robust proteins or processing conditions.
isoelectric precipitation vs heat coagulation Infographic
 libmatt.com
libmatt.com