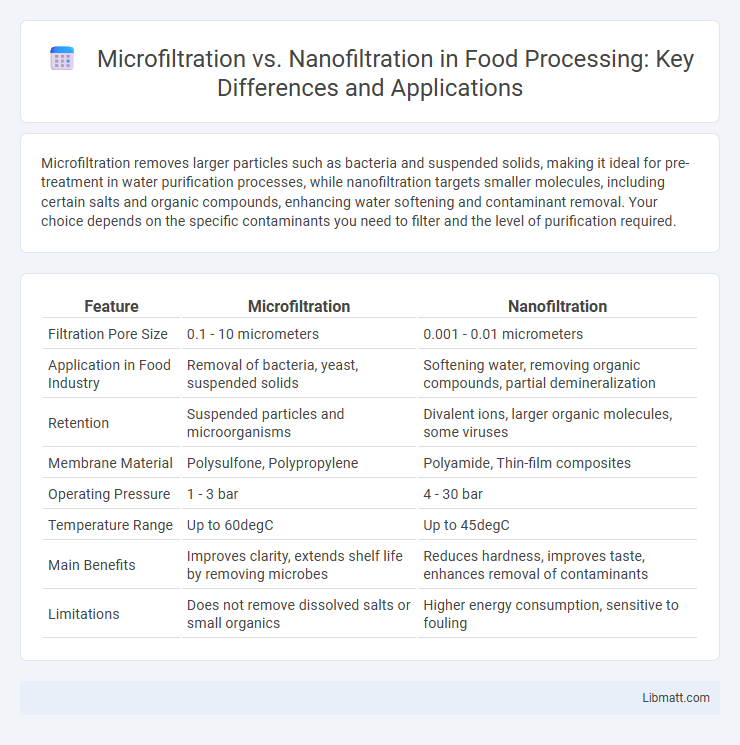
Microfiltration removes larger particles such as bacteria and suspended solids, making it ideal for pre-treatment in water purification processes, while nanofiltration targets smaller molecules, including certain salts and organic compounds, enhancing water softening and contaminant removal. Your choice depends on the specific contaminants you need to filter and the level of purification required.
Table of Comparison
| Feature | Microfiltration | Nanofiltration |
|---|---|---|
| Filtration Pore Size | 0.1 - 10 micrometers | 0.001 - 0.01 micrometers |
| Application in Food Industry | Removal of bacteria, yeast, suspended solids | Softening water, removing organic compounds, partial demineralization |
| Retention | Suspended particles and microorganisms | Divalent ions, larger organic molecules, some viruses |
| Membrane Material | Polysulfone, Polypropylene | Polyamide, Thin-film composites |
| Operating Pressure | 1 - 3 bar | 4 - 30 bar |
| Temperature Range | Up to 60degC | Up to 45degC |
| Main Benefits | Improves clarity, extends shelf life by removing microbes | Reduces hardness, improves taste, enhances removal of contaminants |
| Limitations | Does not remove dissolved salts or small organics | Higher energy consumption, sensitive to fouling |
Introduction to Microfiltration and Nanofiltration
Microfiltration uses membrane filters with pore sizes typically between 0.1 to 10 microns, efficiently removing larger particles such as suspended solids, bacteria, and some viruses from liquids. Nanofiltration features tighter membranes with pore sizes around 1 to 10 nanometers, capable of filtering out smaller molecules, divalent ions, and organic compounds while allowing monovalent ions to pass through. Your water treatment or separation process benefits from choosing between microfiltration for coarse filtration and nanofiltration for more selective purification needs.
Key Differences Between Microfiltration and Nanofiltration
Microfiltration membranes typically have pore sizes ranging from 0.1 to 10 microns, making them ideal for removing larger particles such as suspended solids, bacteria, and some viruses. Nanofiltration membranes feature smaller pores, around 0.001 to 0.01 microns, allowing them to filter out divalent salts, organic molecules, and certain pathogens while retaining monovalent ions. The primary distinction lies in their separation mechanisms and applications, with microfiltration mainly used for particle removal in water treatment and nanofiltration employed for softening and partial desalination processes.
Membrane Structure and Pore Size Comparison
Microfiltration membranes feature larger pores, typically ranging from 0.1 to 10 microns, designed to remove suspended solids, bacteria, and larger particles from water. Nanofiltration membranes have much smaller pores, usually between 1 to 10 nanometers, allowing them to filter out divalent and larger monovalent ions, organic molecules, and some bacteria while maintaining water flow efficiency. Understanding the distinction in pore size and structure is essential for selecting the right filtration technology to meet your specific water purification needs.
Filtration Mechanisms: How Each Process Works
Microfiltration uses membrane pores typically between 0.1 to 10 microns to physically remove particles, bacteria, and suspended solids through size exclusion, relying on a low-pressure filtration process. Nanofiltration operates with smaller pore sizes around 0.001 to 0.01 microns, combining size exclusion with partial removal of divalent and larger monovalent ions through charge effects and diffusion mechanisms. Your choice between microfiltration and nanofiltration depends on the specific contaminants targeted and the level of separation required.
Applications of Microfiltration
Microfiltration is widely applied in water treatment processes to remove suspended solids, bacteria, and large particles from drinking water and wastewater. It is commonly used in food and beverage industries for clarifying liquids, such as milk, beer, and juices, to enhance product quality and safety. Microfiltration also plays a crucial role in pharmaceutical manufacturing by sterilizing solutions without the need for heat.
Applications of Nanofiltration
Nanofiltration is widely applied in water treatment processes, specifically for softening hard water by removing divalent and larger monovalent ions while retaining essential minerals. It is effective in wastewater treatment for removing organic compounds, pesticides, and certain pathogens, making it suitable for industrial and municipal reuse applications. Your choice of nanofiltration technology can improve water quality for beverage production, pharmaceutical processes, and food processing by selectively filtering contaminants while preserving beneficial components.
Advantages and Limitations of Microfiltration
Microfiltration offers advantages such as effective removal of suspended solids, bacteria, and large particles, making it ideal for water treatment and food processing applications. Its lower operational pressure and energy consumption compared to nanofiltration reduce costs and simplify maintenance. However, microfiltration cannot remove dissolved salts or smaller organic molecules, limiting its use in applications requiring fine filtration or desalination.
Advantages and Limitations of Nanofiltration
Nanofiltration offers advantages such as effective removal of divalent and larger monovalent ions, improved permeate quality, and lower operational pressure compared to reverse osmosis, making it energy-efficient for water softening and partial desalination. Limitations include lower rejection rates for monovalent ions like sodium and chloride, vulnerability to membrane fouling, and a higher upfront cost relative to microfiltration systems. This balance makes nanofiltration ideal for applications requiring selective contaminant removal while maintaining moderate energy consumption.
Cost and Energy Efficiency Analysis
Microfiltration typically incurs lower operational costs and energy consumption, making it cost-effective for removing larger particles and microorganisms in water treatment. Nanofiltration, while more expensive upfront and energy-intensive due to higher pressure requirements, excels in removing smaller contaminants such as divalent ions and organic molecules. The choice between microfiltration and nanofiltration should balance treatment goals with budget constraints and energy availability, as nanofiltration can justify higher costs through superior contaminant removal efficiency.
Choosing the Right Filtration Method for Your Needs
Microfiltration and nanofiltration differ primarily in pore size and filtration capabilities, with microfiltration targeting particles between 0.1 to 10 microns and nanofiltration filtering down to 1-10 nanometers, effectively removing smaller contaminants such as certain dissolved solids and organic molecules. Choosing the right filtration method depends on your specific needs, including the level of purification required, the types of contaminants present, and the intended water use. Tailoring the selection to these factors ensures optimal performance and cost-efficiency in water treatment applications.
microfiltration vs nanofiltration Infographic
 libmatt.com
libmatt.com