Silicate glass offers excellent chemical durability and high thermal resistance, making it ideal for everyday uses like windows and containers, while borate glass provides superior thermal shock resistance and enhanced optical properties, often used in laboratory glassware and specialty lenses. Your choice depends on the specific application requirements, balancing strength, thermal stability, and optical clarity.
Table of Comparison
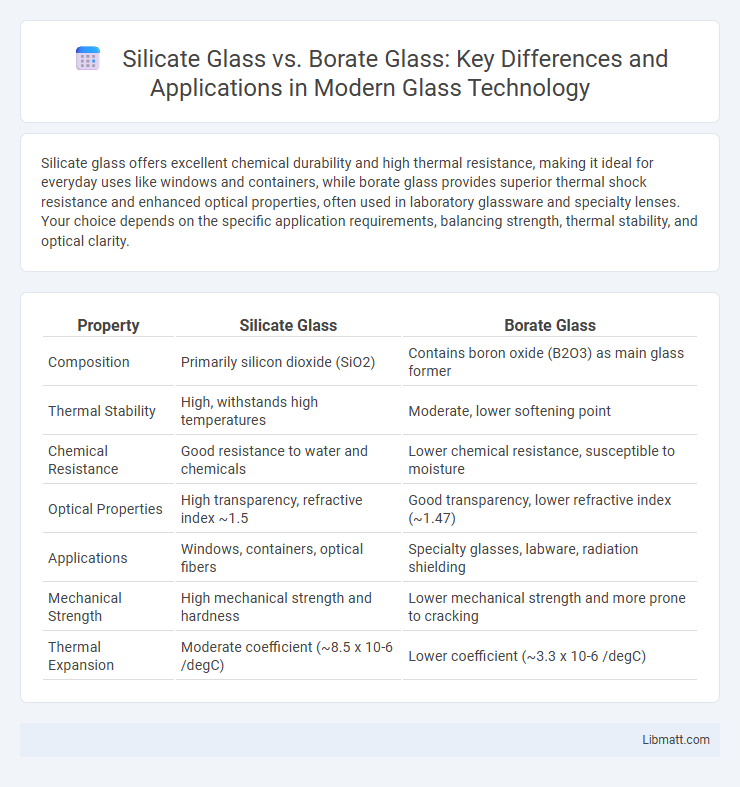
| Property | Silicate Glass | Borate Glass |
|---|---|---|
| Composition | Primarily silicon dioxide (SiO2) | Contains boron oxide (B2O3) as main glass former |
| Thermal Stability | High, withstands high temperatures | Moderate, lower softening point |
| Chemical Resistance | Good resistance to water and chemicals | Lower chemical resistance, susceptible to moisture |
| Optical Properties | High transparency, refractive index ~1.5 | Good transparency, lower refractive index (~1.47) |
| Applications | Windows, containers, optical fibers | Specialty glasses, labware, radiation shielding |
| Mechanical Strength | High mechanical strength and hardness | Lower mechanical strength and more prone to cracking |
| Thermal Expansion | Moderate coefficient (~8.5 x 10-6 /degC) | Lower coefficient (~3.3 x 10-6 /degC) |
Introduction to Silicate Glass and Borate Glass
Silicate glass, composed primarily of silicon dioxide (SiO2), is renowned for its high durability, transparency, and thermal stability, making it ideal for windows, laboratory equipment, and cookware. Borate glass contains boron oxide (B2O3) as a key component, offering enhanced thermal resistance, chemical durability, and lower melting temperatures compared to silicate glass, which suits it well for electronic and optical applications. Understanding the distinct structural properties of silicate and borate glasses helps you select the appropriate material based on performance requirements and environmental conditions.
Chemical Composition Differences
Silicate glass primarily consists of silicon dioxide (SiO2) as its main component, often combined with sodium oxide (Na2O) and calcium oxide (CaO) to improve durability and workability. Borate glass contains a significant amount of boron oxide (B2O3), which imparts unique thermal and chemical resistance properties compared to silicate glass. Your selection between these two types depends on the required thermal stability and chemical resistance for the intended application.
Structural Characteristics
Silicate glass consists primarily of silicon dioxide (SiO2) tetrahedra linked in a three-dimensional network, creating strong covalent bonds that result in high chemical durability and thermal stability. Borate glass features boron oxide (B2O3) units arranged in triangular or tetrahedral coordination, providing a more flexible structure with lower melting points and enhanced optical properties. The structural differences between silicate and borate glasses significantly influence their mechanical strengths, thermal expansion coefficients, and resistance to chemical corrosion.
Manufacturing Processes
Silicate glass manufacturing involves melting silica sand with soda ash and limestone at high temperatures around 1700degC, followed by controlled cooling to form a durable, transparent material primarily used in windows and bottles. Borate glass production requires melting boron oxide with soda lime at slightly lower temperatures of approximately 1150degC, resulting in a heat-resistant, chemically stable glass ideal for laboratory equipment and specialized optical applications. Understanding these distinct manufacturing processes helps you select the appropriate glass type based on thermal and chemical performance requirements.
Physical Properties Comparison
Silicate glass exhibits higher hardness and greater chemical durability compared to borate glass, making it more resistant to scratches and corrosion. Borate glass offers superior thermal shock resistance and lower melting temperatures, enhancing its suitability for applications requiring rapid temperature changes. Density differences are notable, with silicate glass typically denser than borate glass, affecting weight and thermal conductivity in various uses.
Thermal Stability and Resistance
Silicate glass offers superior thermal stability and resistance due to its high melting point and strong silicon-oxygen bonds, making it ideal for applications involving extreme temperature variations. Borate glass, while having lower thermal stability, excels in chemical durability and thermal shock resistance thanks to its unique boron-oxygen network structure. Understanding these properties helps you select the right glass type for your thermal and chemical resistance needs.
Optical Properties Analysis
Silicate glass offers high clarity with excellent light transmission and low dispersion, making it ideal for optical lenses and fiber optics. Borate glass exhibits superior UV transmission and higher refractive indices, enhancing performance in laser and photonic applications. Understanding your specific optical requirements ensures the choice between silicate and borate glass maximizes efficiency and visual precision.
Industrial and Practical Applications
Silicate glass, composed primarily of silicon dioxide, dominates industrial applications such as windows, bottles, and fiberglass due to its durability, chemical resistance, and thermal stability. Borate glass, containing boron oxide, offers superior thermal shock resistance and low melting points, making it ideal for laboratory glassware, medical devices, and specialty optics. Both types are essential in electronics; silicate glass serves as substrates for displays, while borate glass is valued for its optical clarity and radiation shielding in lasers and sensors.
Environmental Impact and Sustainability
Silicate glass, primarily composed of silica, has a high recyclability rate and a low environmental footprint due to abundant raw materials and energy-efficient recycling processes. Borate glass, while offering unique thermal and chemical properties, involves more energy-intensive production and typically lower recyclability, which can lead to greater environmental impact. Choosing silicate glass for your applications promotes sustainability by reducing waste and lowering carbon emissions throughout the lifecycle.
Choosing the Right Glass for Your Needs
Silicate glass offers excellent chemical durability, thermal stability, and mechanical strength, making it ideal for laboratory equipment and everyday containers. Borate glass provides superior thermal shock resistance and lower melting temperatures, suitable for specialized applications like optical fibers and borosilicate cookware. Selecting between silicate and borate glass depends on factors such as thermal performance, chemical resistance, and specific usage requirements.
silicate glass vs borate glass Infographic
 libmatt.com
libmatt.com