Biosimilars are complex, biologically derived medications highly similar to an original biologic drug, while generics are chemically synthesized exact copies of small-molecule drugs. Understanding the differences ensures You make informed choices about effectiveness, safety, and regulatory approval when considering treatment options.
Table of Comparison
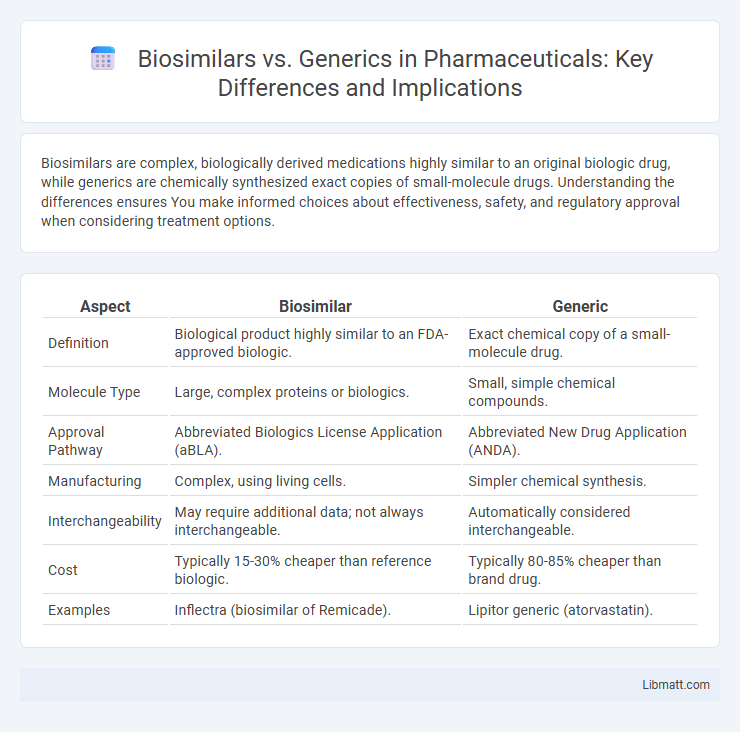
| Aspect | Biosimilar | Generic |
|---|---|---|
| Definition | Biological product highly similar to an FDA-approved biologic. | Exact chemical copy of a small-molecule drug. |
| Molecule Type | Large, complex proteins or biologics. | Small, simple chemical compounds. |
| Approval Pathway | Abbreviated Biologics License Application (aBLA). | Abbreviated New Drug Application (ANDA). |
| Manufacturing | Complex, using living cells. | Simpler chemical synthesis. |
| Interchangeability | May require additional data; not always interchangeable. | Automatically considered interchangeable. |
| Cost | Typically 15-30% cheaper than reference biologic. | Typically 80-85% cheaper than brand drug. |
| Examples | Inflectra (biosimilar of Remicade). | Lipitor generic (atorvastatin). |
Introduction to Biosimilars and Generics
Biosimilars are biologic medical products highly similar to an already approved original biologic, known as the reference product, with no clinically meaningful differences in safety or effectiveness. Generics are chemically synthesized drugs identical in active ingredients, dosage form, strength, and route of administration to brand-name small-molecule drugs. While generics undergo bioequivalence studies to ensure similarity, biosimilars require comprehensive analytical, preclinical, and clinical comparisons due to the complex structure and manufacturing of biologics.
Defining Biosimilars
Biosimilars are biological products highly similar to an original branded biologic but not identical due to the complex nature of living organisms used in production. Unlike generic drugs, which are exact chemical copies of small-molecule medications, biosimilars undergo rigorous analytical and clinical testing to demonstrate no meaningful differences in safety, purity, or efficacy. Understanding biosimilars helps you make informed decisions about treatment options involving biologic therapies.
Understanding Generics
Generics are pharmaceutical drugs that contain the same active ingredients, strength, dosage form, and route of administration as their brand-name counterparts, ensuring therapeutic equivalence and cost-effectiveness. They undergo rigorous FDA approval processes to demonstrate bioequivalence, meaning they deliver the same amount of active substance into a patient's bloodstream in the same time frame. Understanding generics allows you to make informed decisions about effective, affordable medication alternatives without compromising quality or safety.
Key Differences Between Biosimilars and Generics
Biosimilars are complex biologic products highly similar to an original biologic but not identical, due to the intricacies of living cell production, while generics are exact chemical copies of small-molecule drugs. Regulatory pathways for biosimilars involve extensive clinical trials demonstrating comparable safety, efficacy, and immunogenicity, whereas generics require bioequivalence studies proving the same bioavailability as the original drug. Cost and development time differ significantly, with biosimilars demanding higher investment and longer approval periods compared to the relatively straightforward and less expensive generic drug approval process.
Approval Processes: Biosimilars vs. Generics
Biosimilars undergo a stringent approval process involving extensive analytical, preclinical, and clinical studies to demonstrate similarity in safety, efficacy, and immunogenicity to the reference biologic product. Generic drugs follow a more straightforward approval pathway through Abbreviated New Drug Applications (ANDAs), requiring bioequivalence studies without the need for clinical trials. Regulatory agencies, such as the FDA and EMA, apply rigorous standards tailored to the complex nature of biosimilars, making their approval more demanding than that of generics.
Manufacturing Complexities and Challenges
Biosimilars involve complex manufacturing processes due to their derivation from living organisms, requiring stringent control of biological variability and advanced cell culture techniques to ensure consistent product quality. In contrast, generic drugs are chemically synthesized with well-defined structures, allowing for more straightforward replication and standardized quality control. The inherent molecular complexity of biosimilars demands extensive analytical characterization and validation to demonstrate similarity to original biologics, posing significant regulatory and production challenges absent in generic drug manufacturing.
Clinical Efficacy and Safety Considerations
Biosimilars undergo rigorous clinical trials to demonstrate comparable efficacy and safety to their reference biologics, involving extensive immunogenicity and pharmacokinetic assessments. Generics, bioequivalent to small-molecule drugs, require less extensive clinical testing focused mainly on pharmacokinetic bioequivalence studies. Your choice between biosimilars and generics should account for these differences in clinical evaluation to ensure therapeutic interchangeability and patient safety.
Cost Implications and Market Impact
Biosimilars generally have higher development costs and regulatory hurdles than generics, leading to less drastic price reductions but still offering significant cost savings compared to original biologics. Generics, being exact chemical replicas, often cause steeper market price declines, making medications more accessible due to lower production expenses and streamlined approvals. Your choice between biosimilars and generics can affect overall healthcare spending, with biosimilars driving competition in complex biologic markets and generics dominating traditional small-molecule drug sectors.
Regulatory Guidelines for Biosimilars and Generics
Regulatory guidelines for biosimilars require extensive analytical, preclinical, and clinical studies to demonstrate similarity in safety, efficacy, and quality to the reference biologic product, reflecting the complexity of large biologic molecules. Generics undergo bioequivalence testing with the original small-molecule drug, focusing on identical active ingredients, dosage form, and strength, which allows for an abbreviated approval pathway. Your understanding of these regulatory distinctions is crucial for recognizing the rigorous approval process biosimilars face compared to generics.
Future Trends in Biosimilars and Generics
Future trends in biosimilars focus on expanding therapeutic areas, with oncology and autoimmune diseases leading market growth due to increasing biologic drug patents expiring. Generic drug development continues to prioritize cost reduction and accessibility, leveraging advanced manufacturing technologies to enhance production efficiency and regulatory approval processes. Both biosimilars and generics are projected to benefit from digital health integration, enabling improved pharmacovigilance and personalized medicine approaches.
Biosimilar vs Generic Infographic
 libmatt.com
libmatt.com