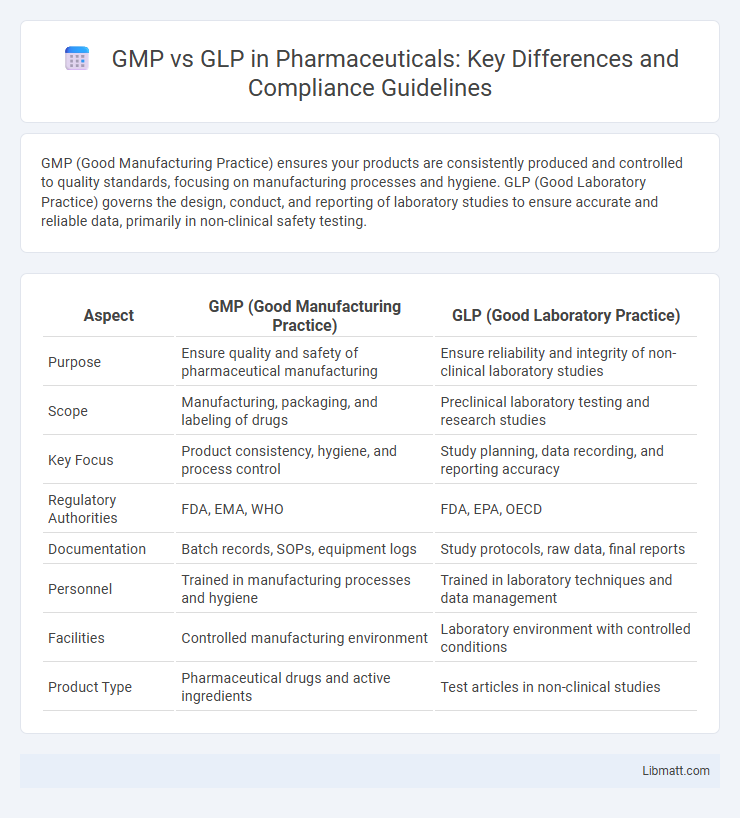
GMP (Good Manufacturing Practice) ensures your products are consistently produced and controlled to quality standards, focusing on manufacturing processes and hygiene. GLP (Good Laboratory Practice) governs the design, conduct, and reporting of laboratory studies to ensure accurate and reliable data, primarily in non-clinical safety testing.
Table of Comparison
| Aspect | GMP (Good Manufacturing Practice) | GLP (Good Laboratory Practice) |
|---|---|---|
| Purpose | Ensure quality and safety of pharmaceutical manufacturing | Ensure reliability and integrity of non-clinical laboratory studies |
| Scope | Manufacturing, packaging, and labeling of drugs | Preclinical laboratory testing and research studies |
| Key Focus | Product consistency, hygiene, and process control | Study planning, data recording, and reporting accuracy |
| Regulatory Authorities | FDA, EMA, WHO | FDA, EPA, OECD |
| Documentation | Batch records, SOPs, equipment logs | Study protocols, raw data, final reports |
| Personnel | Trained in manufacturing processes and hygiene | Trained in laboratory techniques and data management |
| Facilities | Controlled manufacturing environment | Laboratory environment with controlled conditions |
| Product Type | Pharmaceutical drugs and active ingredients | Test articles in non-clinical studies |
Introduction to GMP and GLP
Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) are essential regulatory frameworks ensuring quality and safety in pharmaceutical and laboratory operations. GMP focuses on production processes, guaranteeing products are consistently produced and controlled according to quality standards, while GLP emphasizes the reliability and integrity of non-clinical laboratory studies. Understanding these practices helps you maintain compliance and optimize operational excellence in manufacturing and research environments.
Defining GMP: Good Manufacturing Practice
Good Manufacturing Practice (GMP) establishes stringent guidelines to ensure products are consistently produced and controlled according to quality standards, critical in pharmaceuticals, food, and cosmetics industries. GMP regulations cover all aspects of production, including raw materials, equipment, personnel training, and record-keeping, ensuring product safety and efficacy. Compliance with GMP reduces risks of contamination, mix-ups, and errors, safeguarding consumer health and maintaining regulatory approval.
Defining GLP: Good Laboratory Practice
Good Laboratory Practice (GLP) is a quality system concerned with the organizational process and conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, reported, and archived. GLP ensures the reliability, reproducibility, and integrity of laboratory data through strict adherence to standardized protocols, personnel training, and comprehensive documentation. Unlike Good Manufacturing Practice (GMP), which focuses on the production process and product quality, GLP is primarily concerned with the scientific validity and regulatory compliance of laboratory studies.
Core Objectives: GMP vs GLP
GMP (Good Manufacturing Practice) ensures product quality, safety, and consistency in manufacturing processes, focusing on production and control standards for pharmaceuticals and food. GLP (Good Laboratory Practice) governs laboratory study conduct, emphasizing data integrity, accuracy, and reliability in non-clinical safety testing. Both GMP and GLP establish regulatory frameworks to protect public health but target different stages within product development and manufacturing.
Regulatory Bodies Governing GMP and GLP
The regulatory bodies governing Good Manufacturing Practice (GMP) include the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO), which enforce standards to ensure product safety and quality. Good Laboratory Practice (GLP) is overseen by agencies such as the FDA in the United States and the Organization for Economic Co-operation and Development (OECD), focusing on the integrity of non-clinical laboratory studies. Understanding these regulatory frameworks helps you comply with industry requirements and maintain the credibility of your manufacturing or laboratory processes.
Key Differences between GMP and GLP
GMP (Good Manufacturing Practice) focuses on ensuring the quality and safety of pharmaceutical products through standardized production processes, while GLP (Good Laboratory Practice) emphasizes the integrity and reliability of non-clinical laboratory studies, particularly in safety testing. GMP regulates manufacturing environments, personnel qualifications, and equipment calibration to prevent contamination and ensure consistent product quality. GLP governs laboratory procedures, documentation, and study reporting to ensure data validity and reproducibility in preclinical research.
Compliance Requirements for GMP and GLP
GMP (Good Manufacturing Practice) compliance requirements focus on ensuring product quality and safety through validated processes, rigorous documentation, and stringent facility controls in manufacturing environments. GLP (Good Laboratory Practice) mandates strict protocols for study planning, data collection, and quality assurance to guarantee the integrity and reliability of non-clinical laboratory studies. Both standards emphasize traceability, record-keeping, and regular audits but serve distinct purposes: GMP targets production quality, while GLP centers on data credibility in laboratory research.
Applications in the Pharmaceutical Industry
GMP (Good Manufacturing Practice) ensures the quality and safety of pharmaceutical products by regulating manufacturing processes, equipment, and facility standards. GLP (Good Laboratory Practice) focuses on the integrity and reliability of non-clinical laboratory studies, including safety testing of new drug candidates. Your pharmaceutical development relies on GMP for production compliance and GLP for accurate preclinical research data that supports regulatory submissions.
Impact on Product Quality and Safety
GMP (Good Manufacturing Practice) ensures consistent product quality and safety by regulating manufacturing processes and facility standards, minimizing risks of contamination and errors. GLP (Good Laboratory Practice) focuses on the reliability and integrity of non-clinical laboratory studies, which support product safety assessment and regulatory approval. Understanding both GMP and GLP is essential for maintaining Your product's quality throughout development and production stages.
Choosing Between GMP and GLP: Factors to Consider
Choosing between GMP (Good Manufacturing Practice) and GLP (Good Laboratory Practice) depends on the primary purpose of your operation: GMP ensures product safety and quality in manufacturing processes, while GLP focuses on the integrity and reliability of non-clinical laboratory studies. Regulatory requirements, industry standards, and the product lifecycle stage are critical factors influencing your decision. Assessing whether your focus is on production or research environments will help determine the appropriate compliance framework for your facility.
GMP vs GLP Infographic
 libmatt.com
libmatt.com