Phase 1 clinical trials primarily focus on evaluating the safety, dosage range, and side effects of a new treatment in a small group of healthy volunteers or patients. Phase 3 clinical trials involve a larger population to confirm effectiveness, monitor adverse reactions, and compare the new treatment to standard therapies, ensuring comprehensive data before approval for your use.
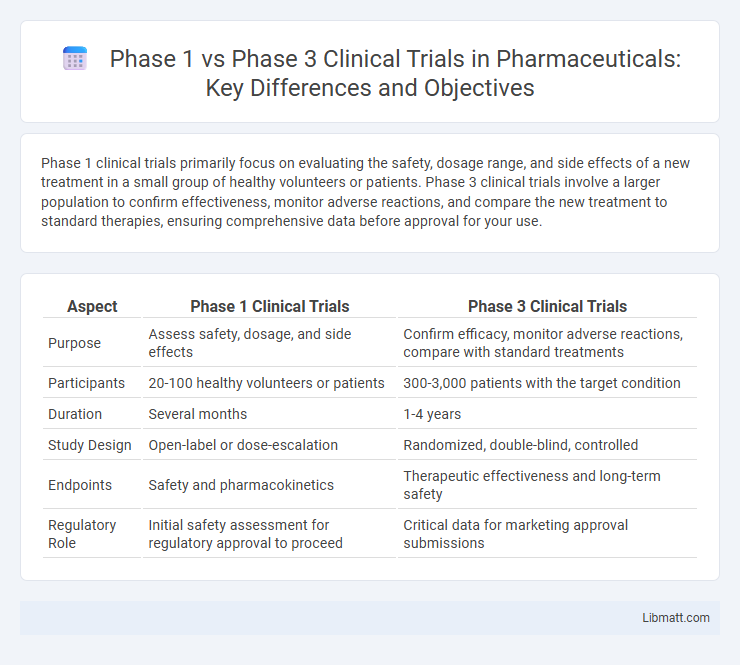
Table of Comparison
| Aspect | Phase 1 Clinical Trials | Phase 3 Clinical Trials |
|---|---|---|
| Purpose | Assess safety, dosage, and side effects | Confirm efficacy, monitor adverse reactions, compare with standard treatments |
| Participants | 20-100 healthy volunteers or patients | 300-3,000 patients with the target condition |
| Duration | Several months | 1-4 years |
| Study Design | Open-label or dose-escalation | Randomized, double-blind, controlled |
| Endpoints | Safety and pharmacokinetics | Therapeutic effectiveness and long-term safety |
| Regulatory Role | Initial safety assessment for regulatory approval to proceed | Critical data for marketing approval submissions |
Introduction to Clinical Trials
Phase 1 clinical trials primarily assess the safety, dosage range, and side effects of a new drug or treatment in a small group of healthy volunteers or patients. Phase 3 clinical trials involve a larger patient population to confirm efficacy, monitor adverse reactions, and compare the new treatment to standard therapies. Understanding these trial phases helps you grasp the rigorous process drugs undergo before approval and widespread use.
Overview of Phases in Clinical Trials
Phase 1 clinical trials primarily assess the safety, dosage range, and side effects of a new treatment using a small group of healthy volunteers or patients. Phase 3 clinical trials involve larger patient populations to confirm the treatment's efficacy, monitor side effects, and compare it to existing standard therapies. Your understanding of these distinct phases is crucial for evaluating the development progress and potential impact of new medical interventions.
Defining Phase 1 Clinical Trials
Phase 1 clinical trials primarily focus on assessing the safety, tolerability, and pharmacokinetics of a new drug in a small group of healthy volunteers or patients. These trials determine the appropriate dosage range and identify side effects, laying the foundation for subsequent trial phases. Understanding Phase 1 is crucial for your drug development journey, as it establishes initial safety profiles before moving to larger, efficacy-focused Phase 3 trials.
Defining Phase 3 Clinical Trials
Phase 3 clinical trials are large-scale studies designed to confirm the efficacy and safety of a drug or treatment established in earlier phases, typically involving hundreds to thousands of participants. These trials compare the new intervention against the current standard treatment or placebo, providing statistically significant data required for regulatory approval. Phase 3 trials assess clinical endpoints such as overall survival, disease progression, and quality of life, serving as the pivotal step before market authorization.
Objectives: Phase 1 vs Phase 3
Phase 1 clinical trials primarily focus on assessing the safety, tolerability, and pharmacokinetics of a new drug in a small group of healthy volunteers or patients. Phase 3 clinical trials aim to confirm the efficacy, monitor adverse reactions, and compare the new treatment to standard therapies in a larger patient population. Your understanding of these objectives is essential for interpreting drug development progress and potential approval.
Participant Selection Differences
Phase 1 clinical trials primarily involve a small group of healthy volunteers selected to assess safety, dosage, and side effects, aiming to determine the appropriate dose range for further testing. Phase 3 trials include a much larger, more diverse patient population who have the disease or condition targeted by the drug, focusing on efficacy and monitoring adverse reactions in real-world scenarios. Participant selection in Phase 3 emphasizes inclusivity of different demographics and comorbidities to ensure generalizability of results compared to the narrowly defined criteria of Phase 1.
Study Design and Methodology Comparison
Phase 1 clinical trials primarily focus on safety and dosage, involving a small number of healthy volunteers with an open-label or dose-escalation design to determine pharmacokinetics and pharmacodynamics. In contrast, Phase 3 trials utilize randomized, double-blind, placebo-controlled methodologies with larger, diverse patient populations to assess efficacy and monitor adverse effects over extended periods. The rigorous statistical analysis and multicenter approach in Phase 3 studies provide the comprehensive data required for regulatory approval.
Key Endpoints: Safety vs Efficacy
Phase 1 clinical trials primarily focus on assessing the safety and tolerability of a new drug, determining its pharmacokinetics and dosage range in a small group of healthy volunteers or patients. Phase 3 clinical trials emphasize evaluating the drug's efficacy and monitoring adverse reactions in a larger patient population to confirm therapeutic benefits and support regulatory approval. Your understanding of these key endpoints is crucial for interpreting clinical trial results and making informed decisions in drug development.
Regulatory and Ethical Considerations
Phase 1 clinical trials prioritize safety, dosing, and pharmacokinetics, requiring strict regulatory approval to ensure participant protection, often involving healthy volunteers under close ethical oversight. Phase 3 trials focus on efficacy and monitoring adverse reactions in larger patient populations, demanding comprehensive regulatory submissions and adherence to rigorous ethical standards for informed consent and risk-benefit balance. Your participation in any phase is safeguarded by institutional review boards (IRBs) that enforce compliance with Good Clinical Practice (GCP) guidelines to uphold patient rights and data integrity.
Impact on Drug Development Process
Phase 1 clinical trials primarily assess drug safety, dosage tolerance, and pharmacokinetics in a small group of healthy volunteers, serving as a critical initial step in drug development. Phase 3 clinical trials evaluate drug efficacy and monitor adverse reactions in large patient populations, providing robust data required for regulatory approval. The transition from Phase 1 to Phase 3 marks a shift from safety and dosage optimization to large-scale confirmation of therapeutic effectiveness, significantly influencing the overall timeline and success of the drug development process.
Phase 1 vs Phase 3 clinical trials Infographic
 libmatt.com
libmatt.com