An active comparator is a treatment known to have therapeutic effects used in clinical trials to directly compare efficacy and safety against a new intervention, whereas a placebo contains no active ingredients and serves as a control to measure the psychological or non-specific effects of a treatment. Choosing between a placebo and an active comparator depends on ethical considerations and the need to demonstrate that Your new treatment is superior or non-inferior to existing therapies.
Table of Comparison
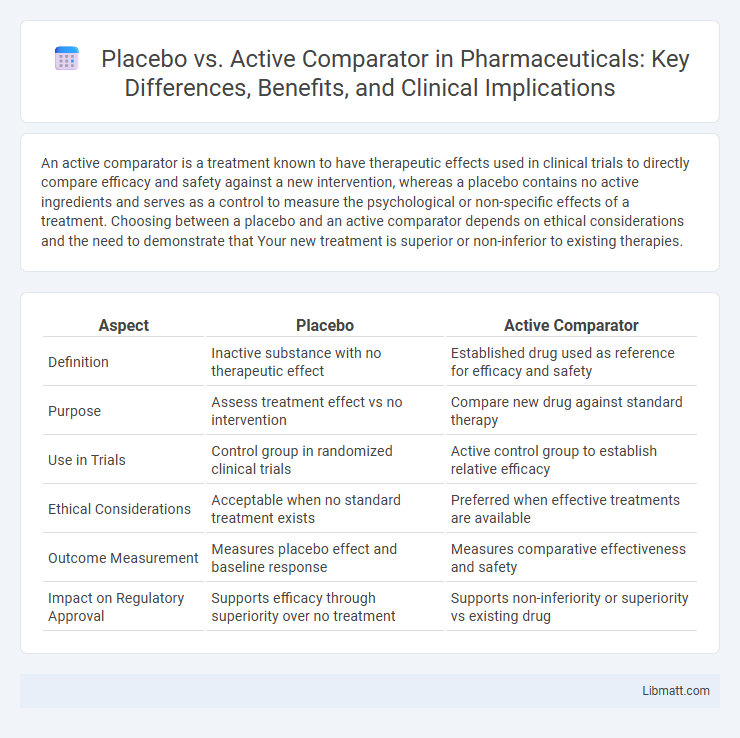
| Aspect | Placebo | Active Comparator |
|---|---|---|
| Definition | Inactive substance with no therapeutic effect | Established drug used as reference for efficacy and safety |
| Purpose | Assess treatment effect vs no intervention | Compare new drug against standard therapy |
| Use in Trials | Control group in randomized clinical trials | Active control group to establish relative efficacy |
| Ethical Considerations | Acceptable when no standard treatment exists | Preferred when effective treatments are available |
| Outcome Measurement | Measures placebo effect and baseline response | Measures comparative effectiveness and safety |
| Impact on Regulatory Approval | Supports efficacy through superiority over no treatment | Supports non-inferiority or superiority vs existing drug |
Introduction to Placebo and Active Comparator
Placebo and active comparator are critical elements in clinical trials used to evaluate a treatment's efficacy and safety. A placebo is an inactive substance designed to resemble the drug being tested, allowing researchers to measure the psychological and physiological effects unrelated to the active treatment. An active comparator involves using an existing approved treatment to compare outcomes directly, ensuring your new therapy demonstrates meaningful benefits over current standards of care.
Definitions: Placebo vs Active Comparator
A placebo is an inert substance or treatment designed to have no therapeutic effect, used to measure the psychological or physiological impact of receiving a treatment. An active comparator refers to an established medication or intervention used as a benchmark in clinical trials to evaluate the efficacy and safety of a new treatment. When assessing your treatment options, understanding these definitions helps clarify the purpose of control groups in clinical research.
Historical Context in Clinical Trials
Placebo controls have been a foundational element in clinical trials since the mid-20th century, serving as a benchmark to measure the efficacy of new treatments against inert substances. Active comparators emerged to address ethical concerns by comparing new interventions directly with existing standard therapies, ensuring patients receive effective care while evaluating relative benefits. Your understanding of these historical shifts enhances the interpretation of trial results and informs evidence-based healthcare decisions.
Benefits of Using Placebo Controls
Using placebo controls allows precise measurement of a treatment's efficacy by isolating its specific therapeutic effects from psychological or external influences. This approach enhances the accuracy of clinical trials, providing clear evidence for regulatory approvals and guiding effective medical decision-making. You gain confidence in the true impact of an intervention without confounding factors from active comparators.
Advantages of Active Comparator Trials
Active comparator trials provide direct evidence of a new treatment's effectiveness relative to existing therapies, enhancing clinical relevance and decision-making for healthcare providers. These trials reduce ethical concerns by ensuring all participants receive active treatment rather than placebo, which is critical in conditions requiring immediate management. They also improve patient recruitment and retention due to the perceived benefit of receiving a proven therapy instead of placebo.
Ethical Considerations and Dilemmas
Ethical considerations in placebo versus active comparator trials center on balancing scientific validity with patient welfare, ensuring participants are not deprived of effective treatment. The dilemma arises when placebo use may withhold proven therapy, risking harm, while active comparators require rigorous selection to match standard care efficacy. Informed consent and risk-benefit assessment are critical to uphold ethical standards in clinical research design.
Regulatory Guidelines and Requirements
Regulatory guidelines mandate that placebo-controlled trials must demonstrate ethical use, ensuring no effective treatment is withheld from participants, particularly in conditions where standard therapies exist. Active comparator trials require rigorous selection of established treatments as benchmarks, with regulatory agencies emphasizing equivalence or non-inferiority study designs to assess therapeutic benefit. Agencies such as the FDA and EMA provide detailed requirements on trial design, informed consent, and outcome measures to ensure reliability and ethical compliance in both placebo and active comparator studies.
Impact on Trial Outcomes and Data Integrity
Using an active comparator instead of a placebo enhances trial outcomes by providing a direct measurement of a new treatment's efficacy against existing standards, ensuring your data reflects real-world clinical relevance. This approach strengthens data integrity through reduced bias and improved participant blinding, leading to more reliable and interpretable results. Ultimately, selecting an active comparator supports robust conclusions regarding the treatment's relative effectiveness and safety profiles.
Selecting the Right Control for Your Study
Selecting the right control for your study is essential to ensure valid and reliable results, where placebo controls help isolate the treatment effect by minimizing bias, especially in conditions with subjective outcomes. Active comparators provide a practical benchmark when ethical concerns prevent withholding effective treatment and help demonstrate comparative efficacy or safety. Your choice depends on study objectives, disease context, and regulatory requirements to optimize clinical relevance and interpretability.
Future Trends in Clinical Trial Design
Emerging trends in clinical trial design emphasize the integration of active comparators over traditional placebo controls to enhance ethical standards and provide more relevant efficacy data. Adaptive trial designs and real-world evidence are increasingly leveraged to compare new treatments directly against established standards, improving decision-making and patient outcomes. By incorporating these innovative methodologies, your future studies can achieve greater scientific validity and regulatory acceptance.
Placebo vs active comparator Infographic
 libmatt.com
libmatt.com