A clinical endpoint directly measures how a patient feels, functions, or survives, providing a clear assessment of treatment benefit. A surrogate endpoint serves as a substitute, using biomarkers or intermediate outcomes to predict clinical benefit, but it may not always accurately reflect actual patient health effects.
Table of Comparison
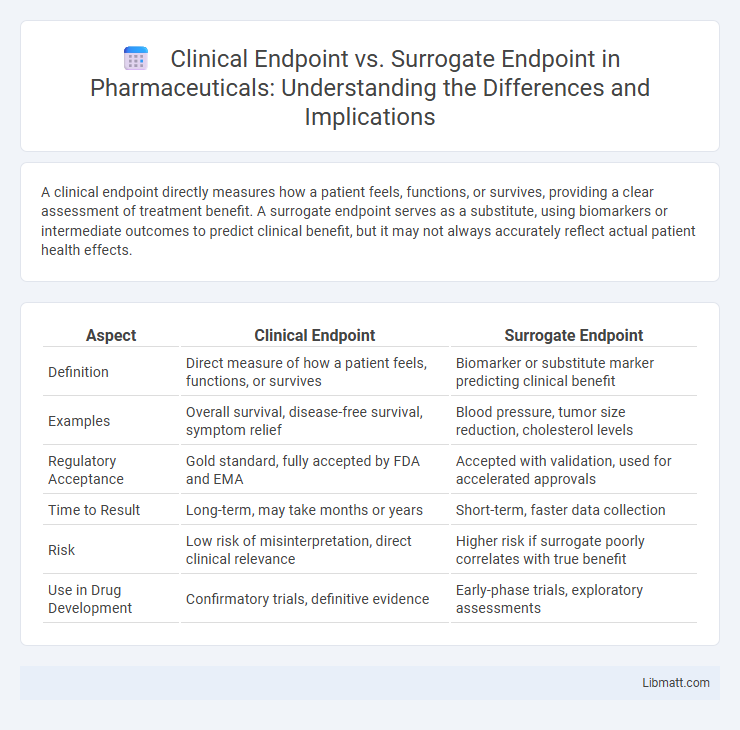
| Aspect | Clinical Endpoint | Surrogate Endpoint |
|---|---|---|
| Definition | Direct measure of how a patient feels, functions, or survives | Biomarker or substitute marker predicting clinical benefit |
| Examples | Overall survival, disease-free survival, symptom relief | Blood pressure, tumor size reduction, cholesterol levels |
| Regulatory Acceptance | Gold standard, fully accepted by FDA and EMA | Accepted with validation, used for accelerated approvals |
| Time to Result | Long-term, may take months or years | Short-term, faster data collection |
| Risk | Low risk of misinterpretation, direct clinical relevance | Higher risk if surrogate poorly correlates with true benefit |
| Use in Drug Development | Confirmatory trials, definitive evidence | Early-phase trials, exploratory assessments |
Introduction to Clinical and Surrogate Endpoints
Clinical endpoints directly measure how a patient feels, functions, or survives, providing definitive evidence of a treatment's impact on health outcomes. Surrogate endpoints are biomarkers or intermediate outcomes used as substitutes for clinical endpoints, offering earlier or more convenient assessments of therapeutic effects. Understanding the distinction between these endpoints is crucial for evaluating your treatment's real-world efficacy and regulatory approval processes.
Defining Clinical Endpoints
Clinical endpoints measure direct effects on how a patient feels, functions, or survives, providing meaningful outcomes such as overall survival or symptom improvement. These endpoints reflect tangible benefits in clinical trials, offering robust evidence of treatment efficacy and safety. Unlike surrogate endpoints, clinical endpoints are not indirect markers but definitive indicators of patient health outcomes.
Understanding Surrogate Endpoints
Surrogate endpoints are biomarkers or intermediate outcomes used in clinical trials as substitutes for direct measures of how a patient feels, functions, or survives. They enable earlier assessment of treatment effects by predicting clinical benefits based on biological or physiological indicators. Understanding surrogate endpoints requires evaluating their validated correlation with true clinical outcomes to ensure reliable and ethical decision-making in drug development.
Key Differences Between Clinical and Surrogate Endpoints
Clinical endpoints directly measure how a patient feels, functions, or survives, making them the most reliable indicators of treatment benefit. Surrogate endpoints, on the other hand, are biomarkers or intermediate outcomes used to predict clinical benefit but do not themselves measure meaningful health outcomes. Understanding these distinctions helps you evaluate the validity and relevance of clinical trial results in medical decision-making.
Importance of Clinical Endpoints in Research
Clinical endpoints directly measure how a patient feels, functions, or survives, making them crucial for assessing the true impact of medical interventions in research. Surrogate endpoints, such as biomarkers or intermediate outcomes, serve as substitutes but may not always reliably predict real-world benefits or risks. Prioritizing clinical endpoints ensures Your study results provide meaningful and clinically relevant information for patient care and regulatory decisions.
Advantages and Limitations of Surrogate Endpoints
Surrogate endpoints offer advantages such as faster trial completion and reduced costs by substituting direct clinical outcomes with biomarkers or intermediate measures. However, their limitations include potential inaccuracies in predicting true clinical benefits, leading to challenges in validating the surrogate's reliability and relevance. Your decision to utilize surrogate endpoints should carefully weigh these trade-offs to ensure meaningful and trustworthy trial results.
Regulatory Perspectives on Endpoint Selection
Regulatory agencies prioritize clinical endpoints because they directly measure how a patient feels, functions, or survives, providing conclusive evidence of treatment benefit. Surrogate endpoints, such as biomarkers or lab measures, are accepted when clinical endpoints are impractical or require long-term observation, but must be scientifically validated to reliably predict clinical outcomes. Your choice of endpoint influences regulatory approval pathways, requiring rigorous justification to demonstrate that surrogate endpoints accurately reflect meaningful clinical benefits.
Impact of Endpoint Choice on Clinical Trial Outcomes
Choosing a clinical endpoint, such as survival rates or disease progression, directly measures the real benefit of a treatment on patient health, ensuring trial outcomes reflect meaningful clinical improvements. Surrogate endpoints, like biomarker changes or tumor size reduction, offer earlier or more accessible indicators but may not always predict long-term patient outcomes accurately. Your selection of endpoint type fundamentally shapes the interpretation, regulatory acceptance, and perceived efficacy of the clinical trial results.
Case Studies: Clinical vs Surrogate Endpoints in Practice
Clinical endpoints directly measure how a patient feels, functions, or survives, providing definitive evidence of treatment benefits, while surrogate endpoints use biomarkers or intermediate outcomes to predict these effects earlier or more efficiently. Case studies in cardiovascular disease reveal that lowering LDL cholesterol (a surrogate) often correlates with reduced heart attack rates (a clinical endpoint), yet some interventions show discordance, emphasizing the need to validate surrogate markers. Understanding the distinction helps ensure your treatment decisions are based on outcomes that truly impact patient health.
Future Trends in Endpoint Selection for Clinical Trials
Future trends in endpoint selection for clinical trials emphasize integrating real-world evidence and digital biomarkers to enhance accuracy and patient-centric outcomes. Machine learning algorithms enable dynamic identification of both clinical and surrogate endpoints, improving predictive validity and trial efficiency. Regulatory agencies increasingly support adaptive designs that incorporate novel endpoints, facilitating faster approval processes and personalized treatment strategies.
Clinical endpoint vs Surrogate endpoint Infographic
 libmatt.com
libmatt.com