A comparator product is used in clinical trials to evaluate the efficacy and safety of a new drug against an existing standard treatment, while a reference product is the original, approved medication that serves as a benchmark for bioequivalence studies. Understanding the differences between comparator and reference products helps you make informed decisions in drug development and regulatory submissions.
Table of Comparison
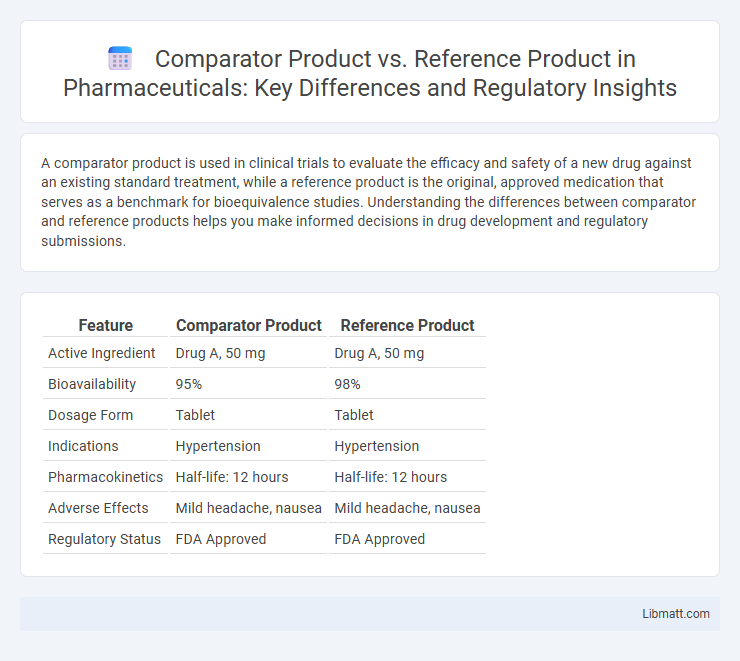
| Feature | Comparator Product | Reference Product |
|---|---|---|
| Active Ingredient | Drug A, 50 mg | Drug A, 50 mg |
| Bioavailability | 95% | 98% |
| Dosage Form | Tablet | Tablet |
| Indications | Hypertension | Hypertension |
| Pharmacokinetics | Half-life: 12 hours | Half-life: 12 hours |
| Adverse Effects | Mild headache, nausea | Mild headache, nausea |
| Regulatory Status | FDA Approved | FDA Approved |
Definition of Comparator and Reference Products
A Comparator Product is a pharmaceutical product used as a benchmark in clinical trials to assess the efficacy and safety of a new drug by comparing it directly against an established Reference Product. The Reference Product is the original, FDA-approved drug with proven safety and efficacy, serving as the standard for bioequivalence studies, particularly in generic drug development. Understanding the distinction between Comparator and Reference Products ensures your clinical evaluations are aligned with regulatory requirements for drug approval.
Key Differences Between Comparator and Reference Products
Comparator products are used in bioequivalence studies to demonstrate similarity to a reference product, which is the original or innovator drug approved by regulatory agencies. Key differences include formulation variations, manufacturing processes, and sourcing, where comparator products might have slight excipients or process changes but must match the reference product's active ingredient profile and therapeutic effect. Regulatory approval for comparator products requires robust data showing no significant differences in safety, efficacy, and quality compared to the reference product.
Importance in Clinical Trials and Studies
Comparator products play a crucial role in clinical trials by serving as benchmarks that enable the assessment of a new product's safety, efficacy, and therapeutic equivalence. Using a well-defined reference product ensures the reliability and validity of study outcomes by providing standardized criteria against which the investigational product is measured. This comparison is essential for regulatory approval processes and helps in establishing bioequivalence, guiding dosage decisions, and confirming the clinical benefits of novel therapies.
Regulatory Requirements for Comparator vs Reference Products
Regulatory requirements for comparator products mandate equivalent quality, safety, and efficacy standards as those for reference products to ensure valid comparative analysis. Both comparator and reference products must comply with stringent guidelines from agencies like the FDA or EMA, including Good Manufacturing Practices (GMP) and stability testing. Documentation must demonstrate bioequivalence or therapeutic equivalence, supporting regulatory approval in clinical trials and marketing authorizations.
Criteria for Selecting a Comparator Product
Criteria for selecting a comparator product include similarity in formulation, bioavailability, and therapeutic indication to the reference product. Regulatory guidelines emphasize matching critical quality attributes and pharmacokinetic profiles to ensure meaningful equivalence. Your choice must align with clinical objectives, ensuring the comparator provides a valid benchmark for efficacy and safety assessments.
Pharmacological Similarities and Differences
Comparator products and reference products often share identical active ingredients, mechanism of action, and therapeutic effects, ensuring comparable pharmacodynamic profiles. Differences may arise from formulation variations, inactive excipients, or manufacturing processes, potentially impacting bioavailability and pharmacokinetics. Comprehensive analytical characterization and clinical bioequivalence studies are critical to substantiate pharmacological similarity and detect any significant disparities affecting efficacy or safety.
Impact on Bioequivalence Assessment
Comparator product selection significantly influences bioequivalence assessment outcomes by ensuring consistent pharmacokinetic parameters between test and reference formulations. Variations in excipient composition or manufacturing processes of the comparator product can affect absorption rates, potentially leading to false conclusions about equivalence. Rigorous comparability of the comparator product to the reference standard is essential to obtain reliable and valid bioequivalence data.
Challenges in Sourcing Comparator and Reference Products
Sourcing comparator and reference products poses significant challenges due to limited availability, regulatory restrictions, and variable supply chain reliability. Manufacturers must navigate complex importation rules and ensure consistent product authenticity to meet regulatory standards. High costs and prolonged lead times further complicate procurement, impacting clinical trial timelines and study outcomes.
Cost Considerations: Comparator vs Reference Product
Cost considerations between comparator and reference products vary significantly, with comparator products often priced lower due to limited clinical data and market exclusivity. Reference products typically incur higher costs driven by extensive research, development investments, and regulatory approval processes. Evaluating total treatment costs requires assessing not only price differences but also impact on healthcare resources and patient outcomes.
Implications for Drug Approval and Market Access
Comparator products play a critical role in demonstrating the safety and efficacy of new drugs when compared to established reference products, which can significantly influence regulatory drug approval decisions. The choice of comparator impacts clinical trial design, data interpretation, and ultimately market access strategies by shaping payer and healthcare provider confidence. Your ability to select an appropriate comparator can accelerate approval timelines and optimize competitive positioning in the pharmaceutical market.
Comparator product vs Reference product Infographic
 libmatt.com
libmatt.com