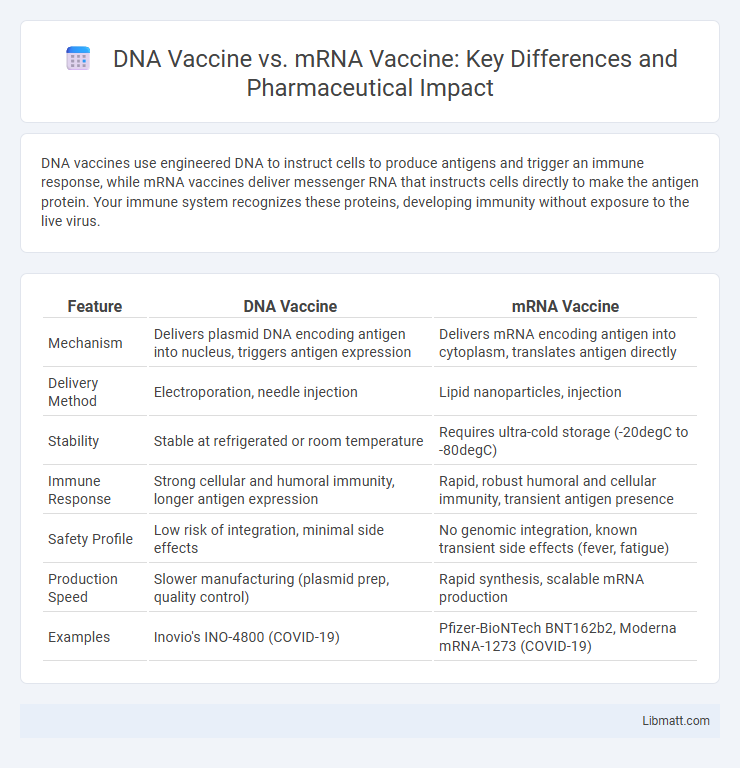
DNA vaccines use engineered DNA to instruct cells to produce antigens and trigger an immune response, while mRNA vaccines deliver messenger RNA that instructs cells directly to make the antigen protein. Your immune system recognizes these proteins, developing immunity without exposure to the live virus.
Table of Comparison
| Feature | DNA Vaccine | mRNA Vaccine |
|---|---|---|
| Mechanism | Delivers plasmid DNA encoding antigen into nucleus, triggers antigen expression | Delivers mRNA encoding antigen into cytoplasm, translates antigen directly |
| Delivery Method | Electroporation, needle injection | Lipid nanoparticles, injection |
| Stability | Stable at refrigerated or room temperature | Requires ultra-cold storage (-20degC to -80degC) |
| Immune Response | Strong cellular and humoral immunity, longer antigen expression | Rapid, robust humoral and cellular immunity, transient antigen presence |
| Safety Profile | Low risk of integration, minimal side effects | No genomic integration, known transient side effects (fever, fatigue) |
| Production Speed | Slower manufacturing (plasmid prep, quality control) | Rapid synthesis, scalable mRNA production |
| Examples | Inovio's INO-4800 (COVID-19) | Pfizer-BioNTech BNT162b2, Moderna mRNA-1273 (COVID-19) |
Introduction to DNA and mRNA Vaccines
DNA vaccines use genetically engineered plasmids to deliver DNA encoding antigens directly into cells, prompting the host to produce the target protein and stimulate an immune response. mRNA vaccines employ messenger RNA molecules that instruct cells to synthesize specific viral proteins, triggering immunity without integrating into the host genome. Both vaccine types harness the body's cellular machinery to generate antigenic proteins and activate adaptive immunity, representing innovative approaches in vaccinology.
How DNA Vaccines Work
DNA vaccines work by introducing a plasmid containing the DNA sequence encoding the antigen into the host cells, where the cellular machinery transcribes the DNA into mRNA. This mRNA is then translated into the target antigen protein, triggering an immune response. Unlike mRNA vaccines, DNA vaccines require delivery methods such as electroporation to enhance cellular uptake and ensure effective antigen expression.
How mRNA Vaccines Work
mRNA vaccines work by delivering synthetic messenger RNA into your cells, instructing them to produce a specific viral protein that triggers an immune response without using live virus particles. This process trains the immune system to recognize and fight the actual virus if exposed, providing effective protection. Unlike DNA vaccines, which require entry into the cell nucleus to deliver genetic instructions, mRNA vaccines operate directly in the cytoplasm, enabling faster protein production and immune activation.
Development and Production Processes
DNA vaccines involve inserting a plasmid containing the DNA sequence encoding the antigen into host cells, which then transcribe and translate it to stimulate an immune response, while mRNA vaccines deliver synthetic mRNA directly into the cytoplasm for protein synthesis. The production of DNA vaccines requires bacterial fermentation to produce plasmids, followed by purification processes, whereas mRNA vaccines are synthesized via in vitro transcription from a DNA template, allowing rapid and scalable manufacturing. Both platforms benefit from cell-free production systems, but mRNA vaccines generally have faster development timelines due to fewer production complexities and no need for cellular uptake by the nucleus.
Mechanism of Immune Response Activation
DNA vaccines deliver plasmid DNA encoding the target antigen into host cells, where it is transcribed into mRNA and subsequently translated into the antigen protein, triggering both cellular and humoral immune responses. mRNA vaccines introduce synthetic mRNA directly into the cytoplasm, enabling immediate translation into antigen proteins, which are then presented on major histocompatibility complex molecules to activate T cells and stimulate antibody production. Both vaccine types promote antigen presentation to dendritic cells, leading to the activation of CD4+ helper T cells and CD8+ cytotoxic T cells, but mRNA vaccines often induce faster protein synthesis and a more robust innate immune signaling cascade.
Safety and Side Effects Comparison
DNA vaccines exhibit a strong safety profile with minimal adverse reactions, primarily causing mild injection site discomfort and low-grade fever. mRNA vaccines, while also generally safe, are linked to transient side effects such as fatigue, headache, muscle pain, and rare cases of myocarditis, especially in young males. Both vaccine types utilize non-live platforms, eliminating risks of infection, but ongoing surveillance continues to refine understanding of their long-term safety and side effect incidence rates.
Efficacy and Effectiveness in Clinical Trials
DNA vaccines have demonstrated robust antigen-specific immune responses with durable cellular immunity in clinical trials, showing efficacy rates typically ranging from 60% to 90% depending on the targeted pathogen. mRNA vaccines exhibit higher efficacy, often exceeding 90%, by inducing strong neutralizing antibody responses and rapid immune activation, as evidenced in COVID-19 clinical trials. Both vaccine platforms show promising safety profiles, but mRNA vaccines generally provide faster scalability and superior effectiveness in preventing symptomatic infections in diverse populations.
Storage and Transportation Requirements
DNA vaccines generally require less stringent storage conditions than mRNA vaccines, often remaining stable at refrigerated temperatures between 2degC and 8degC. mRNA vaccines typically demand ultra-cold storage at temperatures around -70degC, posing significant challenges for transportation and distribution, especially in regions with limited cold chain infrastructure. Ensuring the integrity of your vaccine doses hinges on adhering to these specific storage requirements to maintain efficacy.
Current Approved and Experimental Vaccines
DNA vaccines, such as ZyCoV-D approved in India for COVID-19, use a plasmid containing the DNA sequence encoding the antigen to induce an immune response, whereas mRNA vaccines like Pfizer-BioNTech and Moderna deliver messenger RNA directly encoding the spike protein. Experimental DNA vaccines under clinical trials include Inovio's INO-4800 for COVID-19 and several candidates targeting cancers and infectious diseases. mRNA vaccines have gained wider emergency use authorization globally, with numerous mRNA platforms rapidly advanced for infectious diseases beyond COVID-19, including influenza and cytomegalovirus.
Future Prospects and Innovations
DNA vaccines offer longer stability at room temperature and simpler manufacturing processes, making them promising for global distribution and rapid response to emerging diseases. mRNA vaccines enable faster design modifications and have demonstrated high efficacy in recent pandemics, driving innovations in lipid nanoparticle delivery systems and personalized cancer therapies. Ongoing research explores combining DNA and mRNA technologies to enhance immune responses and expand applications across infectious diseases and oncology.
DNA Vaccine vs mRNA Vaccine Infographic
 libmatt.com
libmatt.com