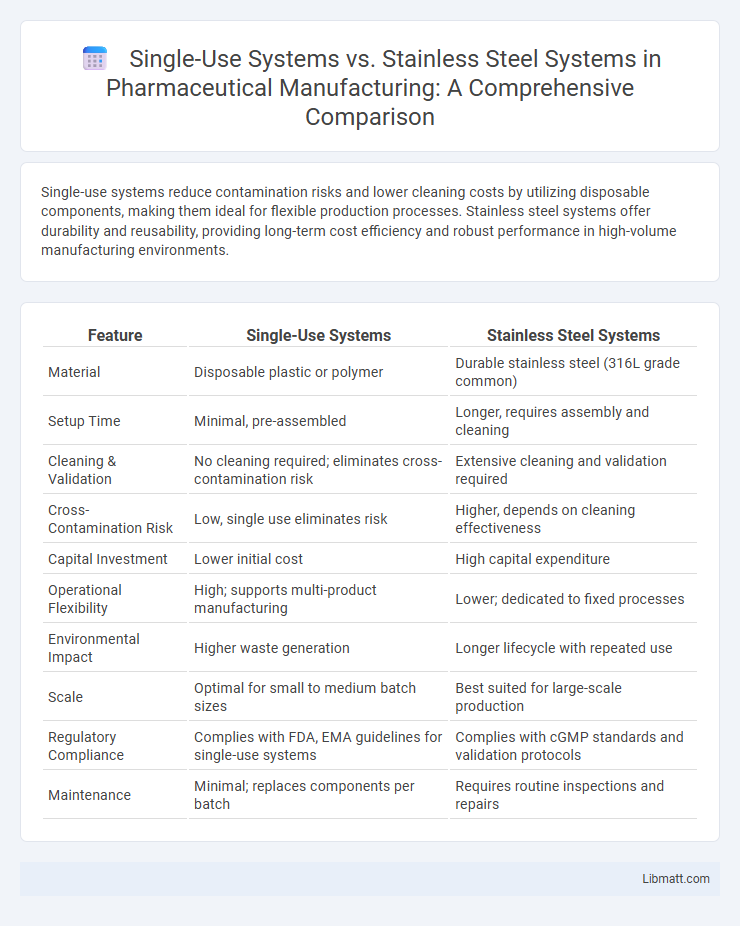
Single-use systems reduce contamination risks and lower cleaning costs by utilizing disposable components, making them ideal for flexible production processes. Stainless steel systems offer durability and reusability, providing long-term cost efficiency and robust performance in high-volume manufacturing environments.
Table of Comparison
| Feature | Single-Use Systems | Stainless Steel Systems |
|---|---|---|
| Material | Disposable plastic or polymer | Durable stainless steel (316L grade common) |
| Setup Time | Minimal, pre-assembled | Longer, requires assembly and cleaning |
| Cleaning & Validation | No cleaning required; eliminates cross-contamination risk | Extensive cleaning and validation required |
| Cross-Contamination Risk | Low, single use eliminates risk | Higher, depends on cleaning effectiveness |
| Capital Investment | Lower initial cost | High capital expenditure |
| Operational Flexibility | High; supports multi-product manufacturing | Lower; dedicated to fixed processes |
| Environmental Impact | Higher waste generation | Longer lifecycle with repeated use |
| Scale | Optimal for small to medium batch sizes | Best suited for large-scale production |
| Regulatory Compliance | Complies with FDA, EMA guidelines for single-use systems | Complies with cGMP standards and validation protocols |
| Maintenance | Minimal; replaces components per batch | Requires routine inspections and repairs |
Introduction to Bioprocessing Systems
Single-use systems offer flexible, disposable bioprocessing solutions that reduce contamination risk and minimize cleaning validation efforts, making them ideal for small to medium-scale production. Stainless steel systems provide durable, reusable infrastructure suitable for large-scale, high-volume manufacturing, ensuring robust process control and long-term cost efficiency. The choice between these bioprocessing systems depends on factors such as production scale, process complexity, and investment in operational infrastructure.
Overview of Single-Use Systems
Single-use systems consist of pre-sterilized, disposable components designed for one-time use in biopharmaceutical manufacturing, reducing cross-contamination risks and minimizing cleaning and sterilization requirements. These systems enhance operational flexibility and decrease turnaround time between production batches, making them ideal for multi-product facilities or smaller-scale operations. Your choice of single-use technology can lead to significant cost savings in facility design and maintenance compared to traditional stainless steel systems.
Overview of Stainless Steel Systems
Stainless steel systems offer robust durability and superior chemical resistance, making them ideal for large-scale industrial processes requiring repeated use and sterilization. Their structural integrity supports high-pressure and high-temperature conditions, ensuring consistent product quality in pharmaceutical and bioprocessing applications. You benefit from long-term cost efficiency despite the higher initial investment, especially when handling complex production cycles.
Key Differences Between Single-Use and Stainless Steel
Single-use systems offer disposable components that minimize cross-contamination risks and reduce cleaning validation requirements, making them ideal for flexible and small-batch bioprocessing. Stainless steel systems provide durability and cost-efficiency for long-term, large-scale production, with higher initial capital investment and more rigorous cleaning protocols. The choice depends on production scale, process complexity, and regulatory compliance needs in biomanufacturing.
Cost Considerations: Initial and Operational Expenses
Single-use systems typically offer lower initial capital expenditures due to reduced need for large-scale infrastructure, enabling faster setup and flexibility in production. Operational expenses may decrease as these systems minimize cleaning, sterilization, and validation costs compared to stainless steel systems, which require extensive maintenance and utilities. Your choice impacts overall cost efficiency, balancing upfront investment against long-term operational savings.
Flexibility and Scalability in Manufacturing
Single-use systems offer superior flexibility in manufacturing by enabling rapid changeovers and reducing cleaning requirements, ideal for multi-product facilities and small batch production. Stainless steel systems provide scalability through robust design suited for large-scale, continuous production but require longer downtime for cleaning and validation. Choosing the right system impacts your manufacturing agility and capacity to meet evolving production demands efficiently.
Cleaning, Sterilization, and Turnaround Times
Single-use systems eliminate the need for cleaning and sterilization, drastically reducing turnaround times by allowing immediate use after disposal. Stainless steel systems require thorough cleaning and sterilization processes, including validation to prevent contamination, which significantly extends downtime. Your production efficiency can improve with single-use systems due to minimized cleaning steps, but stainless steel offers durability and repeated use in long-term operations.
Environmental Impact and Sustainability
Single-use systems typically generate more plastic waste, posing challenges for environmental sustainability, while stainless steel systems reduce waste through repeated use and durability. However, stainless steel systems require substantial water and energy for cleaning and sterilization, increasing their overall carbon footprint. Lifecycle assessments often highlight the trade-off between single-use convenience and the resource intensity of maintaining stainless steel operations.
Regulatory and Compliance Perspectives
Single-use systems offer advantages in regulatory compliance by minimizing cross-contamination risks and reducing cleaning validation requirements, aligning with stringent FDA and EMA guidelines. Stainless steel systems demand comprehensive cleaning and sterilization protocols, necessitating extensive validation to meet cGMP standards. Both systems require robust documentation and adherence to quality management systems to ensure consistent compliance with current Good Manufacturing Practices.
Choosing the Right Solution for Your Facility
Single-use systems offer flexibility, reduced cross-contamination risk, and lower upfront costs, making them ideal for facilities with multiple product batches and smaller production scales. Stainless steel systems provide durability, high chemical resistance, and excellent cleaning capabilities, suited for large-scale, continuous operations requiring stringent sterilization. Evaluating production volume, budget constraints, cleaning validation requirements, and product changeover frequency are critical in selecting the optimal system for your facility.
Single-use systems vs Stainless steel systems Infographic
 libmatt.com
libmatt.com