UV-Vis spectrometry measures the absorption of ultraviolet and visible light by molecules to determine their concentration and electronic structure, while fluorescence spectrometry detects the emitted light from excited molecules, providing higher sensitivity and specificity for trace analysis. You can choose UV-Vis for rapid, straightforward sensing, or fluorescence spectrometry when needing enhanced detection limits and molecular characterization.
Table of Comparison
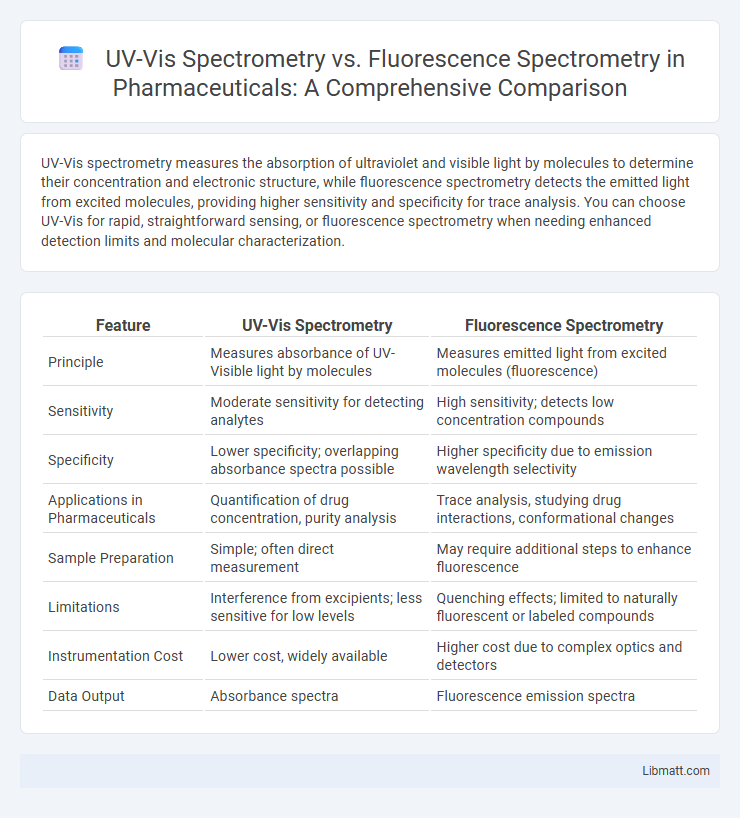
| Feature | UV-Vis Spectrometry | Fluorescence Spectrometry |
|---|---|---|
| Principle | Measures absorbance of UV-Visible light by molecules | Measures emitted light from excited molecules (fluorescence) |
| Sensitivity | Moderate sensitivity for detecting analytes | High sensitivity; detects low concentration compounds |
| Specificity | Lower specificity; overlapping absorbance spectra possible | Higher specificity due to emission wavelength selectivity |
| Applications in Pharmaceuticals | Quantification of drug concentration, purity analysis | Trace analysis, studying drug interactions, conformational changes |
| Sample Preparation | Simple; often direct measurement | May require additional steps to enhance fluorescence |
| Limitations | Interference from excipients; less sensitive for low levels | Quenching effects; limited to naturally fluorescent or labeled compounds |
| Instrumentation Cost | Lower cost, widely available | Higher cost due to complex optics and detectors |
| Data Output | Absorbance spectra | Fluorescence emission spectra |
Overview of UV-Vis Spectrometry
UV-Vis spectrometry measures the absorption of ultraviolet and visible light by molecules, providing quantitative information about electronic transitions and molecular structure. This technique is widely used for analyzing concentrations of compounds in solution, identifying functional groups, and studying reaction kinetics. Its broad applicability and straightforward operation make UV-Vis spectrometry a fundamental tool in chemical, biological, and environmental analysis.
Fundamentals of Fluorescence Spectrometry
Fluorescence spectrometry measures the emission of light by a substance after it absorbs photons, typically in the ultraviolet or visible range, highlighting molecular electronic transitions. Unlike UV-Vis spectrometry, which records absorbance based on light attenuation, fluorescence emphasizes emitted light intensity, offering higher sensitivity for detecting trace amounts of fluorescent compounds. This technique relies on excitation and emission wavelengths, quantum yield, and fluorescence lifetime to analyze the molecular environment and structural properties.
Key Principles: Absorption vs Emission
UV-Vis spectrometry measures the absorption of ultraviolet or visible light by molecules, quantifying the amount of light absorbed at specific wavelengths as electrons transition between energy levels. Fluorescence spectrometry detects the emission of light from molecules that have absorbed higher-energy photons and then released lower-energy photons during relaxation to the ground state. The fundamental distinction lies in UV-Vis monitoring absorbed light intensity, whereas fluorescence analyzes emitted light intensity, providing complementary information about molecular structure and environment.
Instrumentation and Setup Differences
UV-Vis spectrometry uses a light source, monochromator, sample holder, and photodetector to measure absorbance of UV and visible light by a sample, while fluorescence spectrometry includes an excitation source, emission monochromator, sample chamber, and highly sensitive photomultiplier tube or CCD detector to capture emitted light. UV-Vis requires simpler alignment and fewer optical components, whereas fluorescence spectrometry demands precise positioning to separate excitation and emission paths and minimize background noise. Your choice depends on desired sensitivity and specificity, as fluorescence spectrometry typically offers higher sensitivity but involves more complex instrumentation and calibration.
Types of Samples and Applications
UV-Vis spectrometry analyzes a wide range of samples including liquids, solids, and gases by measuring absorbance or transmittance of UV and visible light, ideal for concentration determination and reaction kinetics in pharmaceuticals and environmental monitoring. Fluorescence spectrometry is suited for samples containing fluorescent molecules or labels, commonly used in biological and chemical research for detecting trace elements, biomolecules, and assessing molecular interactions with high sensitivity. Your choice depends on the sample's fluorescent properties and the desired application, such as quantitative analysis via UV-Vis or sensitive detection and imaging through fluorescence techniques.
Sensitivity and Detection Limits
UV-Vis spectrometry provides moderate sensitivity, detecting analytes in micromolar to millimolar concentration ranges, making it suitable for many routine analyses. Fluorescence spectrometry offers significantly higher sensitivity, often reaching nanomolar to picomolar detection limits due to its selective emission measurement and lower background noise. For applications requiring ultra-trace detection, your choice of fluorescence spectrometry can enhance analytical precision beyond what UV-Vis methods typically achieve.
Quantitative Analysis: Accuracy and Precision
UV-Vis spectrometry provides reliable quantitative analysis with high accuracy in measuring absorbance related to concentration, but its precision can be limited by overlapping absorption bands and baseline drifts. Fluorescence spectrometry offers superior sensitivity and precision due to its ability to detect low concentrations with minimal background interference, making it ideal for trace analysis. Your choice between these techniques should consider the sample's properties and required detection limits to optimize both accuracy and precision in quantitative measurements.
Advantages and Limitations of Each Method
UV-Vis spectrometry offers rapid, cost-effective analysis of molecular absorption, ideal for quantifying concentration and studying electronic transitions in various samples, but it struggles with low sensitivity and interference from overlapping spectra. Fluorescence spectrometry provides exceptional sensitivity and selectivity by detecting emitted light from excited molecules, enabling trace-level detection and detailed structural insights, yet it requires fluorescent samples or labeling and is susceptible to quenching effects. Both techniques complement each other, with UV-Vis favored for broad screening and fluorescence preferred for highly sensitive and specific applications.
Common Challenges and Interference
UV-Vis spectrometry often faces challenges such as baseline drift, stray light, and overlapping absorption bands, which can interfere with accurate quantification. Fluorescence spectrometry encounters issues like photobleaching, inner filter effects, and signal quenching due to environmental factors or sample impurities. Your choice between these techniques should consider the sample's optical properties and potential interferences to ensure reliable analytical results.
Choosing the Right Technique for Your Analysis
UV-Vis spectrometry measures the absorbance of ultraviolet and visible light by a sample, making it ideal for quantifying concentration and studying electronic transitions in molecules with strong chromophores. Fluorescence spectrometry detects the emission of light from excited molecules returning to their ground state, offering higher sensitivity and selectivity for trace analysis and studying fluorescent compounds. Your choice depends on the required sensitivity, sample type, and the specific molecular information sought for accurate and effective analysis.
UV-Vis spectrometry vs fluorescence spectrometry Infographic
 libmatt.com
libmatt.com