HVAC validation ensures your heating, ventilation, and air conditioning systems meet regulatory standards for airflow, temperature, and contamination control, crucial in sterile or controlled environments. Water system validation focuses on verifying water quality, flow rates, pressure, and microbial control to maintain safety and compliance in industrial and pharmaceutical applications.
Table of Comparison
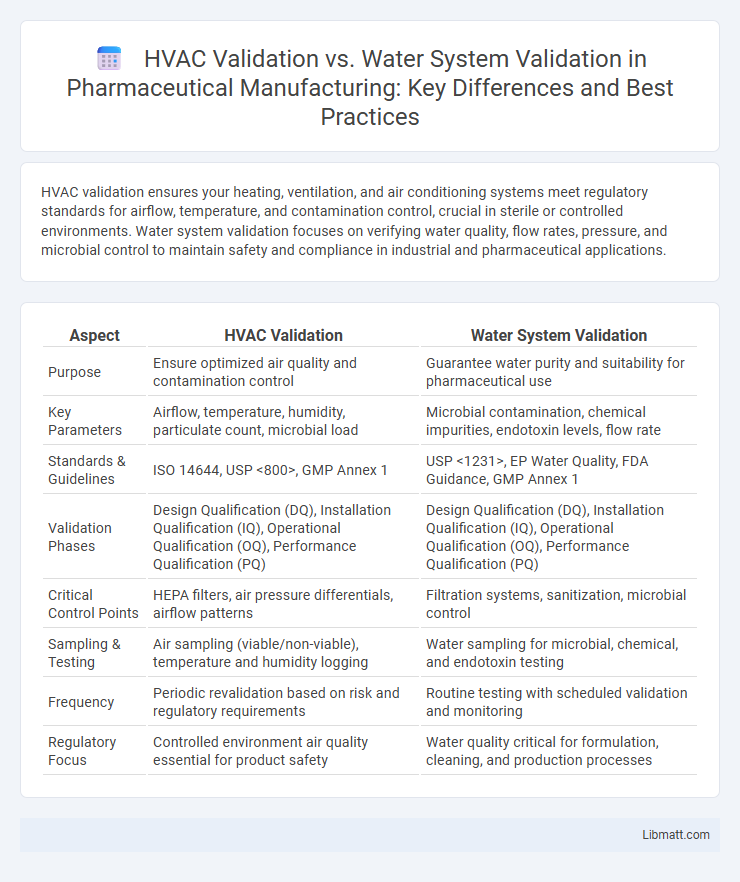
| Aspect | HVAC Validation | Water System Validation |
|---|---|---|
| Purpose | Ensure optimized air quality and contamination control | Guarantee water purity and suitability for pharmaceutical use |
| Key Parameters | Airflow, temperature, humidity, particulate count, microbial load | Microbial contamination, chemical impurities, endotoxin levels, flow rate |
| Standards & Guidelines | ISO 14644, USP <800>, GMP Annex 1 | USP <1231>, EP Water Quality, FDA Guidance, GMP Annex 1 |
| Validation Phases | Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ) | Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), Performance Qualification (PQ) |
| Critical Control Points | HEPA filters, air pressure differentials, airflow patterns | Filtration systems, sanitization, microbial control |
| Sampling & Testing | Air sampling (viable/non-viable), temperature and humidity logging | Water sampling for microbial, chemical, and endotoxin testing |
| Frequency | Periodic revalidation based on risk and regulatory requirements | Routine testing with scheduled validation and monitoring |
| Regulatory Focus | Controlled environment air quality essential for product safety | Water quality critical for formulation, cleaning, and production processes |
Introduction to HVAC and Water System Validation
HVAC validation ensures heating, ventilation, and air conditioning systems maintain controlled environments critical for pharmaceutical and manufacturing processes, focusing on parameters such as airflow, temperature, and humidity. Water system validation verifies the purity, quality, and microbial safety of water used in production, emphasizing parameters like microbial contamination, chemical composition, and endotoxin levels. Both validations adhere to regulatory standards such as USP, FDA, and EMA guidelines to guarantee product safety and compliance.
Purpose and Importance of Validation in Critical Systems
HVAC validation ensures controlled air quality, temperature, and humidity levels to prevent contamination in critical environments, directly impacting product safety and regulatory compliance. Water system validation verifies microbial control, chemical composition, and system integrity to guarantee the purity of water used in pharmaceutical and manufacturing processes. Both validations uphold critical system reliability, protect product quality, and meet stringent industry standards like FDA and USP.
Regulatory Requirements for HVAC and Water Systems
Regulatory requirements for HVAC systems emphasize compliance with ASHRAE standards and FDA guidelines to ensure proper airflow, temperature control, and contamination prevention in controlled environments. Water system validation must adhere to USP <1231> and EPA regulations to guarantee water purity, microbial safety, and chemical compliance in pharmaceutical and industrial applications. Both HVAC and water systems require rigorous documentation, routine monitoring, and periodic revalidation to meet industry-specific regulatory expectations.
Key Differences Between HVAC and Water System Validation
HVAC validation primarily ensures air quality, temperature control, and system performance meet regulatory standards, while water system validation focuses on water purity, microbial control, and contamination prevention. The key differences lie in the critical parameters tested: HVAC emphasizes airflow, particulate levels, and pressure differentials, whereas water system validation centers on microbial counts, chemical composition, and temperature. Understanding these distinctions helps you implement appropriate validation protocols tailored to your specific system requirements.
Core Components of HVAC Validation
Core components of HVAC validation include airflow measurement, temperature and humidity control, filter integrity testing, and system pressure verification to ensure environmental conditions meet regulatory standards. These elements are critical for maintaining contamination control and product quality in pharmaceutical or cleanroom environments. Your validation process must also incorporate routine calibration of sensors and documentation to guarantee consistent system performance.
Essential Elements of Water System Validation
Water system validation requires precise monitoring of microbial control, endotoxin levels, and water purity to ensure safety and compliance in pharmaceutical and manufacturing environments. Critical parameters such as total organic carbon (TOC), conductivity, and temperature must be consistently validated to maintain water quality within defined limits. Unlike HVAC validation, which centers on airflow and particle control, water system validation focuses on these essential elements to safeguard Your processes from contamination risks.
Validation Protocols and Documentation
HVAC validation protocols require detailed testing of airflow, temperature, humidity, and pressure differentials to ensure compliance with cleanroom standards, supported by comprehensive documentation including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Water system validation protocols emphasize microbiological sampling, chemical analysis, and system sanitization to verify water purity and system integrity, accompanied by rigorous batch records and validation reports. Both validation processes mandate precise documentation to demonstrate regulatory compliance, traceability, and maintenance of critical quality attributes.
Common Challenges in HVAC vs. Water System Validation
Validating HVAC and water systems involves distinct challenges due to their differing operational parameters and contamination risks. HVAC validation often struggles with maintaining consistent airflow and ensuring microbial control in air quality, while water system validation faces issues with biofilm formation and chemical residual monitoring. Your approach must address these unique hurdles by implementing tailored monitoring protocols and validation criteria specific to each system's critical control points.
Risk Assessment Strategies for Each System
Risk assessment strategies for HVAC validation prioritize airflow patterns, contamination control, temperature, and humidity parameters to ensure safe and compliant air quality in controlled environments. Water system validation focuses on microbial contamination risks, chemical exposure, water quality parameters, and system integrity to prevent biofilm formation and maintain sterile conditions. Tailored risk assessments incorporate system-specific hazards, regulatory standards, and critical control points to optimize validation protocols and mitigate potential failures.
Best Practices and Industry Standards for Ongoing Validation
HVAC validation and water system validation both require adherence to industry standards such as ASHRAE guidelines for HVAC and USP <1231> for water systems to ensure ongoing compliance and performance. Best practices include routine monitoring, scheduled preventative maintenance, and real-time data logging to detect deviations promptly and maintain system integrity. Implementing a risk-based approach enhances validation efforts by targeting critical control points aligned with regulatory expectations from agencies like FDA and EMA.
HVAC validation vs water system validation Infographic
 libmatt.com
libmatt.com