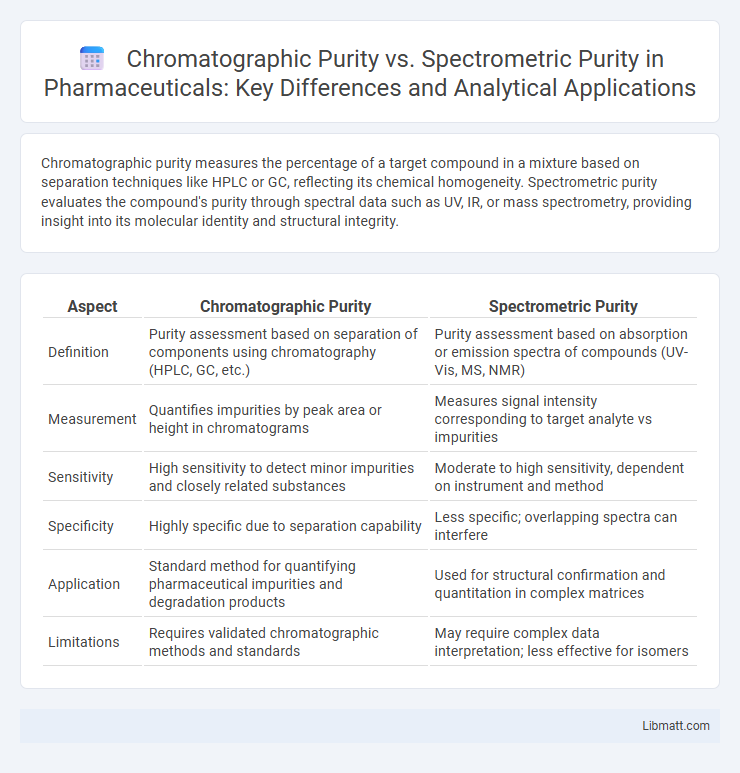
Chromatographic purity measures the percentage of a target compound in a mixture based on separation techniques like HPLC or GC, reflecting its chemical homogeneity. Spectrometric purity evaluates the compound's purity through spectral data such as UV, IR, or mass spectrometry, providing insight into its molecular identity and structural integrity.
Table of Comparison
| Aspect | Chromatographic Purity | Spectrometric Purity |
|---|---|---|
| Definition | Purity assessment based on separation of components using chromatography (HPLC, GC, etc.) | Purity assessment based on absorption or emission spectra of compounds (UV-Vis, MS, NMR) |
| Measurement | Quantifies impurities by peak area or height in chromatograms | Measures signal intensity corresponding to target analyte vs impurities |
| Sensitivity | High sensitivity to detect minor impurities and closely related substances | Moderate to high sensitivity, dependent on instrument and method |
| Specificity | Highly specific due to separation capability | Less specific; overlapping spectra can interfere |
| Application | Standard method for quantifying pharmaceutical impurities and degradation products | Used for structural confirmation and quantitation in complex matrices |
| Limitations | Requires validated chromatographic methods and standards | May require complex data interpretation; less effective for isomers |
Introduction to Purity Assessment in Analytical Chemistry
Purity assessment in analytical chemistry evaluates the presence of impurities in a sample to ensure accuracy and reliability of results. Chromatographic purity measures the proportion of the target compound by separating components based on their interactions with the stationary and mobile phases, providing insight into individual impurity levels. Spectrometric purity quantifies purity through spectral data, such as UV-Vis or mass spectra, by comparing the signal intensity of the desired compound against total absorbance or signal, allowing Your accurate identification of compounds in complex mixtures.
Defining Chromatographic Purity
Chromatographic purity refers to the percentage of a target compound in a sample as determined by chromatographic techniques, such as high-performance liquid chromatography (HPLC), which separate components based on their interaction with the stationary and mobile phases. This method quantifies impurities by detecting and measuring all chromatographically resolvable components, providing high specificity and sensitivity for purity assessment. Chromatographic purity is critical in pharmaceutical quality control to ensure the absence of impurities that may not be detected by spectrometric purity methods.
Understanding Spectrometric Purity
Spectrometric purity refers to the assessment of a compound's purity based on its UV-Vis absorbance spectrum, where impurities are detected through variations in absorbance at specific wavelengths. Unlike chromatographic purity, which separates components physically, spectrometric purity provides a direct evaluation of the sample's optical characteristics without separation. Understanding spectrometric purity helps you identify subtle impurities that might not be resolved chromatographically, ensuring more accurate purity analysis.
Core Principles: Chromatography vs. Spectrometry
Chromatographic purity measures the presence and proportion of components in a mixture by separating them based on their interaction with a stationary phase and mobile phase, providing detailed insight into individual compound concentrations. Spectrometric purity evaluates the overall composition by analyzing the sample's spectral response, such as absorbance or mass-to-charge ratios, reflecting the sample's bulk properties without physically separating components. Understanding these core principles helps you select the most accurate method for assessing your sample's purity depending on whether separation or spectral characterization is paramount.
Techniques for Measuring Chromatographic Purity
Chromatographic purity is determined using techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and thin-layer chromatography (TLC), which separate components of a mixture to identify and quantify impurities. These methods rely on differences in retention times and peak area integration to assess the percentage of the desired compound relative to impurities. Your choice of technique depends on the sample type, with HPLC being preferred for complex mixtures due to its high resolution and sensitivity.
Techniques for Assessing Spectrometric Purity
Techniques for assessing spectrometric purity primarily involve mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy, providing detailed molecular-level identification of impurities. High-resolution mass spectrometry (HRMS) detects minor molecular ion peaks corresponding to contaminants, while NMR reveals structural anomalies through unexpected chemical shifts or coupling patterns. These methods ensure precise quantification and qualification of purity, surpassing chromatographic methods in identifying non-chromophoric or co-eluting impurities.
Key Differences Between Chromatographic and Spectrometric Purity
Chromatographic purity measures the proportion of the main component relative to impurities by separating compounds based on their retention times using techniques like HPLC or GC, providing detailed impurity profiles. Spectrometric purity relies on spectral data obtained from methods such as UV-Vis or mass spectrometry to estimate purity by analyzing absorption or ion patterns, offering faster but less detailed impurity information. The key differences lie in their analytical approaches: chromatographic purity offers higher resolution and quantification of individual impurities, while spectrometric purity provides rapid assessments with broader spectral data but limited impurity discrimination.
Advantages and Limitations of Each Approach
Chromatographic purity offers precise separation and quantification of individual components within a mixture, making it highly effective for identifying impurities and ensuring sample consistency. Spectrometric purity provides rapid assessment by measuring overall molecular characteristics, facilitating quick screening but often lacking specificity for complex mixtures. You can leverage chromatographic techniques for detailed analysis while relying on spectrometric methods for efficient, broader purity evaluations, balancing accuracy with speed depending on your requirements.
Applications in Pharmaceutical and Chemical Analysis
Chromatographic purity is crucial in pharmaceutical and chemical analysis for separating complex mixtures and quantifying individual components with high precision, ensuring drug safety and efficacy. Spectrometric purity provides rapid identification and confirmation of molecular structures by analyzing spectral data, facilitating quality control and impurity profiling. Your choice between chromatographic and spectrometric methods depends on the specific application, such as impurity detection sensitivity or compound identification requirements.
Choosing the Right Purity Assessment Method
Choosing the right purity assessment method depends on the specific analytical requirements and sample characteristics, with chromatographic purity offering detailed separation and quantification of individual impurities through techniques like HPLC or GC. Spectrometric purity provides a rapid assessment based on the absorbance or emission properties of the substance, suitable for confirming compound identity and detecting gross contamination but less effective for complex mixtures. Selecting chromatographic purity is ideal for comprehensive impurity profiling, while spectrometric purity is preferred for quick screening and confirmation in quality control processes.
Chromatographic purity vs Spectrometric purity Infographic
 libmatt.com
libmatt.com