Preclinical studies focus on laboratory and animal testing to evaluate the safety and biological activity of a drug before it reaches humans. Your understanding of clinical studies should include their role in assessing a drug's efficacy and safety in human volunteers under controlled conditions.
Table of Comparison
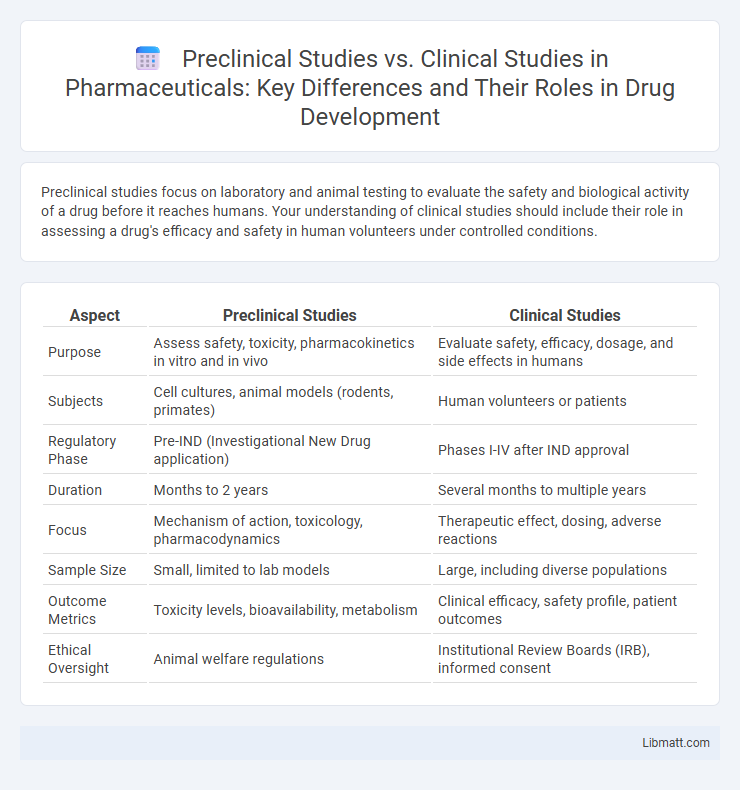
| Aspect | Preclinical Studies | Clinical Studies |
|---|---|---|
| Purpose | Assess safety, toxicity, pharmacokinetics in vitro and in vivo | Evaluate safety, efficacy, dosage, and side effects in humans |
| Subjects | Cell cultures, animal models (rodents, primates) | Human volunteers or patients |
| Regulatory Phase | Pre-IND (Investigational New Drug application) | Phases I-IV after IND approval |
| Duration | Months to 2 years | Several months to multiple years |
| Focus | Mechanism of action, toxicology, pharmacodynamics | Therapeutic effect, dosing, adverse reactions |
| Sample Size | Small, limited to lab models | Large, including diverse populations |
| Outcome Metrics | Toxicity levels, bioavailability, metabolism | Clinical efficacy, safety profile, patient outcomes |
| Ethical Oversight | Animal welfare regulations | Institutional Review Boards (IRB), informed consent |
Introduction to Preclinical and Clinical Studies
Preclinical studies involve laboratory and animal research designed to assess the safety and biological activity of a drug before testing in humans, serving as a critical foundation for clinical trials. Clinical studies are human trials conducted in phases to evaluate the drug's safety, efficacy, dosage, and side effects under controlled conditions. Understanding the transition from preclinical to clinical studies is essential for ensuring your treatments meet regulatory standards and clinical effectiveness.
Key Differences Between Preclinical and Clinical Studies
Preclinical studies involve laboratory and animal testing to evaluate the safety, toxicity, and biological activity of a drug before it is administered to humans. Clinical studies are conducted with human participants to assess the drug's efficacy, optimal dosage, side effects, and overall safety in controlled phases. Your understanding of these key differences is crucial for navigating drug development and regulatory approval processes.
Purpose and Objectives of Preclinical Studies
Preclinical studies are designed to evaluate the safety, efficacy, and biological activity of a drug or treatment before human trials begin, focusing on toxicity, pharmacodynamics, and pharmacokinetics. These studies use in vitro systems and animal models to identify potential risks and therapeutic effects, ensuring the candidate is suitable for progression to clinical trials. The primary objective is to generate comprehensive data supporting the initiation of Phase I clinical trials while minimizing potential harm to human subjects.
Purpose and Objectives of Clinical Studies
Clinical studies aim to evaluate the safety, efficacy, and optimal dosage of new drugs or treatments in human subjects, building on the safety data established during preclinical studies. These studies are designed to answer specific research questions through phases I to IV, focusing on assessing therapeutic effects, side effects, and long-term outcomes. Your participation in clinical trials contributes to advancing medical knowledge and developing treatments that can improve patient care and public health.
Regulatory Requirements for Preclinical vs Clinical Research
Regulatory requirements for preclinical studies mandate comprehensive safety pharmacology, toxicology, and pharmacokinetics data to support Investigational New Drug (IND) applications, ensuring initial human exposure is justified. Clinical research regulations demand adherence to Good Clinical Practice (GCP) guidelines, Institutional Review Board (IRB) approvals, informed consent, and rigorous monitoring to protect human subjects while generating reliable efficacy and safety data. Your understanding of these distinct regulatory landscapes is crucial for successful drug development and compliance with agencies like the FDA and EMA.
Study Design Considerations: Preclinical vs Clinical
Preclinical studies emphasize controlled laboratory experiments using in vitro and animal models to assess safety, efficacy, and pharmacokinetics before human testing, focusing on rigid protocols for reproducibility and ethical compliance. Clinical studies involve phased trial designs (Phase I-IV) with human participants, prioritizing patient selection criteria, randomization, blinding, and regulatory oversight to evaluate therapeutic outcomes and adverse effects in diverse populations. Both study designs require careful consideration of endpoints, statistical power, and ethical guidelines to ensure valid, translatable results from laboratory findings to human applications.
Ethical Considerations in Preclinical and Clinical Studies
Preclinical studies prioritize animal welfare and adhere to strict regulatory guidelines to minimize suffering and ensure humane treatment, involving ethical review boards for protocol approval. Clinical studies emphasize informed consent, patient safety, and risk-benefit analysis, overseen by Institutional Review Boards (IRBs) to protect human participants. Both study types require rigorous ethical considerations to balance scientific advancement with the rights and welfare of subjects involved.
Challenges and Limitations in Preclinical Research
Preclinical studies face significant challenges such as limited translatability of animal model results to human physiology, variability in experimental design, and ethical concerns regarding animal use. These limitations often result in high attrition rates when moving from preclinical to clinical phases, increasing costs and delaying drug development timelines. Addressing these challenges requires improved model relevance and standardized protocols to enhance predictability and reproducibility.
Challenges and Limitations in Clinical Research
Clinical research faces significant challenges including recruitment difficulties, high costs, and ethical constraints that limit the scope and pace of studies. Unlike preclinical studies conducted in controlled laboratory settings, clinical trials must navigate patient variability, regulatory requirements, and unforeseen adverse effects. You must carefully design and manage clinical studies to address these limitations and ensure reliable, applicable results for human health.
Impact of Preclinical and Clinical Studies on Drug Development
Preclinical studies provide essential data on drug safety, efficacy, and pharmacokinetics using in vitro and in vivo models, forming the foundation for human trials. Clinical studies evaluate drug effects in humans through phased trials, determining therapeutic benefits, dosing, and potential side effects, which are crucial for regulatory approval. The integration of preclinical and clinical data accelerates drug development by minimizing risks and optimizing candidate selection for successful market entry.
Preclinical studies vs Clinical studies Infographic
 libmatt.com
libmatt.com