Preclinical research involves laboratory and animal studies to evaluate the safety and efficacy of new treatments before they are tested in humans. Clinical research focuses on human trials to assess the therapeutic effects, dosage, and side effects, ultimately guiding Your medical decisions and treatment options.
Table of Comparison
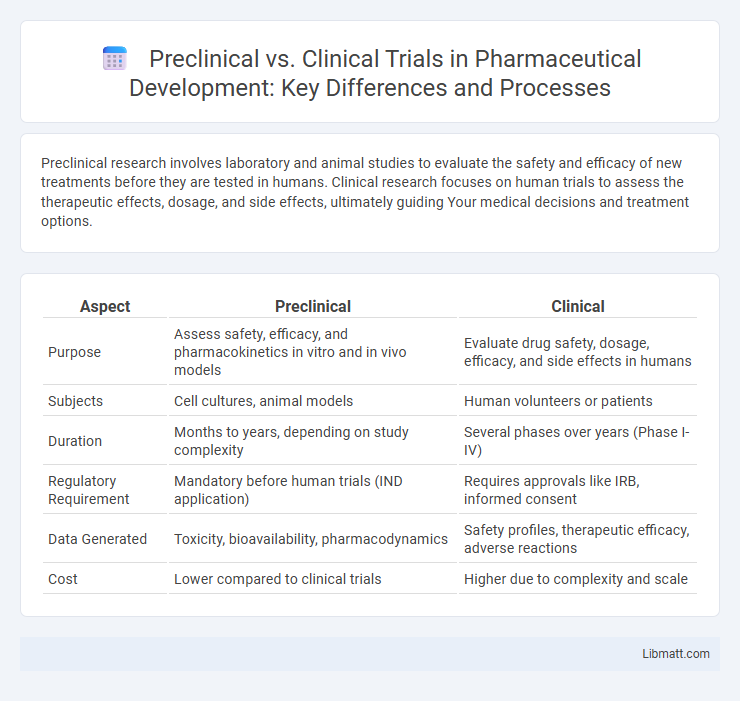
| Aspect | Preclinical | Clinical |
|---|---|---|
| Purpose | Assess safety, efficacy, and pharmacokinetics in vitro and in vivo models | Evaluate drug safety, dosage, efficacy, and side effects in humans |
| Subjects | Cell cultures, animal models | Human volunteers or patients |
| Duration | Months to years, depending on study complexity | Several phases over years (Phase I-IV) |
| Regulatory Requirement | Mandatory before human trials (IND application) | Requires approvals like IRB, informed consent |
| Data Generated | Toxicity, bioavailability, pharmacodynamics | Safety profiles, therapeutic efficacy, adverse reactions |
| Cost | Lower compared to clinical trials | Higher due to complexity and scale |
Introduction to Preclinical and Clinical Research
Preclinical research involves laboratory and animal studies that assess the safety and biological activity of a drug before testing in humans, providing essential data on toxicity and pharmacokinetics. Clinical research follows, with human trials divided into phases I through IV to evaluate safety, efficacy, dosage, and long-term effects in diverse populations. Your understanding of the distinctions between these stages is crucial for navigating drug development and regulatory approvals.
Key Differences Between Preclinical and Clinical Studies
Preclinical studies primarily involve laboratory and animal testing to evaluate the safety and efficacy of a potential treatment before it reaches humans, focusing on pharmacology, toxicology, and dosage. Clinical studies, conducted in multiple phases with human participants, assess the treatment's safety, efficacy, dosage, and side effects in real-world conditions. Understanding these key differences ensures your research or treatment development aligns with regulatory standards and progresses efficiently from laboratory discovery to patient care.
Objectives of Preclinical Research
Preclinical research aims to evaluate the safety, efficacy, and pharmacokinetics of new drug candidates before human trials, using in vitro and animal models. This stage focuses on identifying potential toxicities and therapeutic effects to ensure that only promising compounds advance to clinical phases. Your understanding of these objectives is crucial for interpreting the rationale behind drug development and regulatory approval processes.
Phases of Clinical Trials
The phases of clinical trials are critical steps in medical research, starting with Phase I, which assesses safety and dosage in a small group of healthy volunteers. Phase II evaluates the efficacy and side effects in a larger patient population, while Phase III confirms effectiveness, monitors adverse reactions, and compares the treatment to standard therapies in diverse populations. Your understanding of these phases ensures informed decisions about new medical interventions transitioning from preclinical studies to human testing.
Regulatory Requirements in Preclinical vs Clinical Stages
Regulatory requirements in the preclinical stage demand thorough toxicology and pharmacokinetics studies to demonstrate initial safety before human trials, as mandated by agencies like the FDA and EMA. During the clinical stage, regulations focus extensively on Good Clinical Practice (GCP) compliance, informed consent, and monitoring adverse events across Phase I to Phase III trials. Your adherence to these distinct regulatory frameworks ensures ethical standards and patient safety throughout the drug development process.
Common Models and Methods in Preclinical Testing
Preclinical testing employs a variety of common models and methods, including in vitro cell cultures, animal models like rodents, and advanced techniques such as organ-on-a-chip systems to evaluate drug safety and efficacy before human trials. These models provide critical biological insights and help identify potential toxicities, pharmacokinetics, and pharmacodynamics profiles. Understanding these preclinical methods ensures Your clinical research can advance with a solid foundation for human testing.
Patient Involvement: Clinical Trials Explained
Clinical trials involve direct patient participation to evaluate the safety and efficacy of new treatments, while preclinical studies rely on laboratory and animal testing without patient involvement. Your role as a patient in clinical trials includes informed consent, adherence to protocol, and reporting outcomes that help determine a drug's real-world impact. Understanding patient involvement is crucial to appreciating how clinical research advances medical knowledge beyond the preclinical phase.
Challenges Faced During Preclinical and Clinical Development
Preclinical development faces challenges such as establishing relevant animal models, ensuring drug safety, and predicting human responses accurately, which often delay progression. Clinical development encounters difficulties including patient recruitment, managing diverse population responses, regulatory compliance, and high costs associated with large-scale trials. Your ability to navigate these obstacles is crucial for successful translation from laboratory research to approved therapies.
Importance of Translational Research
Translational research bridges the gap between preclinical findings and clinical applications, ensuring laboratory discoveries effectively inform patient treatments. Your understanding of this process highlights how animal models and in vitro studies must accurately predict clinical outcomes to improve therapeutic interventions. Emphasizing translational research accelerates the development of safe and effective medical solutions by aligning experimental data with human biology.
Future Trends in Preclinical and Clinical Studies
Emerging technologies like artificial intelligence and advanced imaging are revolutionizing preclinical studies by enhancing the accuracy of disease models and speeding up drug discovery. In clinical research, personalized medicine and real-world evidence are shaping future trends by tailoring treatments to individual patient profiles and improving trial outcomes. Your access to innovative methodologies will accelerate the transition from preclinical findings to effective clinical therapies.
Preclinical vs clinical Infographic
 libmatt.com
libmatt.com