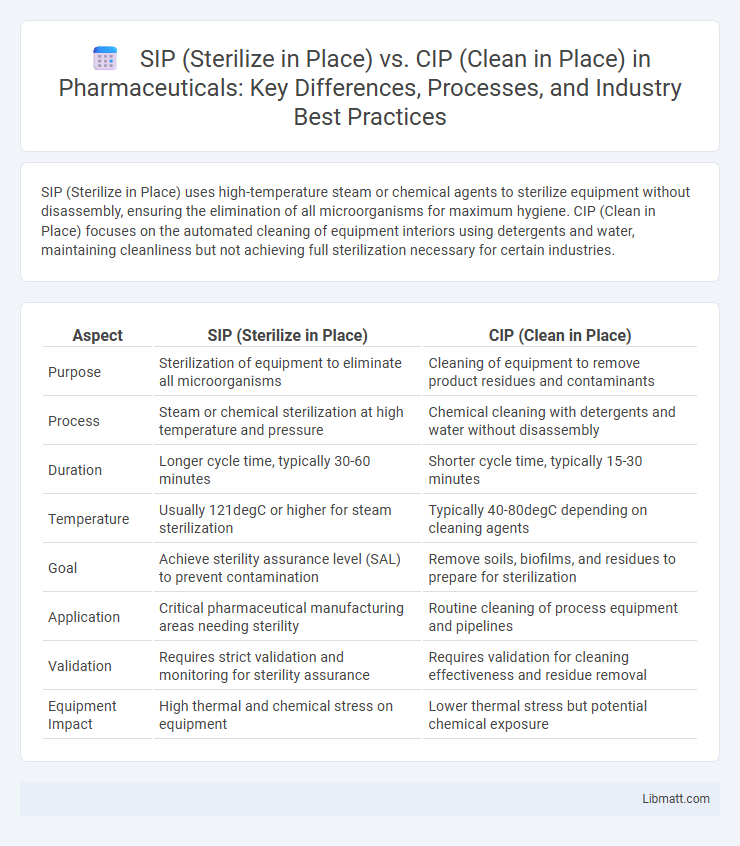
SIP (Sterilize in Place) uses high-temperature steam or chemical agents to sterilize equipment without disassembly, ensuring the elimination of all microorganisms for maximum hygiene. CIP (Clean in Place) focuses on the automated cleaning of equipment interiors using detergents and water, maintaining cleanliness but not achieving full sterilization necessary for certain industries.
Table of Comparison
| Aspect | SIP (Sterilize in Place) | CIP (Clean in Place) |
|---|---|---|
| Purpose | Sterilization of equipment to eliminate all microorganisms | Cleaning of equipment to remove product residues and contaminants |
| Process | Steam or chemical sterilization at high temperature and pressure | Chemical cleaning with detergents and water without disassembly |
| Duration | Longer cycle time, typically 30-60 minutes | Shorter cycle time, typically 15-30 minutes |
| Temperature | Usually 121degC or higher for steam sterilization | Typically 40-80degC depending on cleaning agents |
| Goal | Achieve sterility assurance level (SAL) to prevent contamination | Remove soils, biofilms, and residues to prepare for sterilization |
| Application | Critical pharmaceutical manufacturing areas needing sterility | Routine cleaning of process equipment and pipelines |
| Validation | Requires strict validation and monitoring for sterility assurance | Requires validation for cleaning effectiveness and residue removal |
| Equipment Impact | High thermal and chemical stress on equipment | Lower thermal stress but potential chemical exposure |
Introduction to SIP and CIP
SIP (Sterilize in Place) and CIP (Clean in Place) are essential procedures in pharmaceutical and food processing industries to maintain hygiene and prevent contamination. SIP involves sterilizing equipment and piping systems using steam or chemical agents without disassembly, ensuring complete microbial deactivation. CIP focuses on cleaning the internal surfaces of equipment by circulating detergents and rinsing agents, preparing your system for effective sterilization during SIP.
Defining SIP: What Is Sterilize in Place?
Sterilize in Place (SIP) is a process designed to sterilize equipment and piping systems without disassembly, using high-temperature steam or chemical agents to eliminate all microorganisms and spores. SIP ensures aseptic conditions critical in pharmaceutical, food, and biotechnology industries, maintaining sterility throughout production. Unlike Clean in Place (CIP), which removes contaminants and debris, SIP achieves complete sterilization by destroying microbial life to prevent contamination in sterile manufacturing environments.
Understanding CIP: What Is Clean in Place?
Clean in Place (CIP) is a method that enables the cleaning of interior surfaces of pipes, vessels, and equipment without disassembly, utilizing automated cycles of detergents, rinsing, and sanitizers. CIP systems are essential for maintaining hygiene in industries like food and beverage, dairy, and pharmaceuticals, ensuring contamination-free production while minimizing downtime. Your facility can benefit from CIP by enhancing operational efficiency and ensuring consistent cleaning standards without the need for manual intervention.
Key Differences Between SIP and CIP
SIP (Sterilize in Place) uses high-temperature steam or chemical agents to eliminate all microorganisms on equipment, ensuring sterility, while CIP (Clean in Place) involves automated circulation of detergents and sanitizers to remove organic and inorganic residues without disassembly. SIP is crucial for aseptic processing environments requiring complete sterility, whereas CIP primarily focuses on cleaning to maintain hygiene and prevent contamination before sterilization. Your choice between SIP and CIP depends on whether sterilization or thorough cleaning aligns best with your operational requirements and regulatory standards.
Core Components of SIP Systems
Core components of SIP (Sterilize in Place) systems include steam generators, sterilization chambers, temperature and pressure sensors, and advanced control units to ensure precise sterilization cycles. These systems utilize high-pressure saturated steam to eliminate microbial contaminants, making sterile processing essential in pharmaceutical and food industries. In contrast to CIP (Clean in Place), which primarily focuses on removing residues and soils using detergents and rinsing agents, SIP targets complete sterilization, ensuring equipment is free from viable microorganisms.
Essential Elements of CIP Systems
CIP (Clean in Place) systems feature essential elements including tanks for detergents and rinsing fluids, pump(s) to circulate cleaning solutions, valves to control fluid flow, and sensors for monitoring temperature and conductivity. Automated control panels ensure precise timing and sequence, optimizing cleaning efficiency and safety for your equipment. Unlike SIP (Sterilize in Place), which focuses on microbial inactivation using high heat or chemicals, CIP primarily targets removing soil and residues to maintain hygienic conditions.
Process Workflow: SIP vs CIP
SIP (Sterilize in Place) utilizes high-temperature steam or chemical sterilants to eliminate all microbial life within equipment without disassembly, ensuring aseptic conditions in pharmaceutical or food processing systems. CIP (Clean in Place) involves circulating detergents and rinse water through equipment to remove soil and residues, preparing surfaces for subsequent sterilization or use. Your choice between SIP and CIP depends on the required hygiene level and process workflow, as SIP is a sterilization step following CIP's cleaning process.
Industry Applications for SIP and CIP
SIP (Sterilize in Place) is widely utilized in pharmaceutical, biotechnology, and food processing industries to ensure aseptic conditions by eliminating all microbial life from production equipment without disassembly. CIP (Clean in Place) is predominantly employed in beverage, dairy, and brewery sectors to remove product residues and biofilms from pipelines and tanks, maintaining hygiene and preventing contamination. Both SIP and CIP are crucial for compliance with GMP regulations and enhancing production efficiency in sanitary manufacturing environments.
Advantages and Limitations of SIP Compared to CIP
SIP (Sterilize In Place) offers the advantage of effectively eliminating all microbial life, including spores, ensuring complete sterilization of equipment, while CIP (Clean In Place) primarily focuses on removing residues and reducing microbial load without full sterilization. SIP systems require higher energy consumption and longer cycle times compared to CIP, which can be more cost-effective and faster for routine cleaning. Your choice between SIP and CIP depends on the criticality of sterility needed, with SIP being essential for pharmaceutical and aseptic processes and CIP suitable for less stringent hygienic cleaning in food and beverage industries.
Choosing Between SIP and CIP: Decision Factors
Choosing between SIP (Sterilize in Place) and CIP (Clean in Place) depends on several key factors, including the required level of microbial control, equipment configuration, and production process complexity. SIP is essential when complete sterilization is necessary to eliminate all forms of microbial life, especially in pharmaceutical and biotech industries, while CIP is suitable for routine cleaning where sterilization is not critical. Your decision should also consider downtime, chemical usage, and regulatory compliance to optimize operational efficiency.
SIP (Sterilize in Place) vs CIP (Clean in Place) Infographic
 libmatt.com
libmatt.com