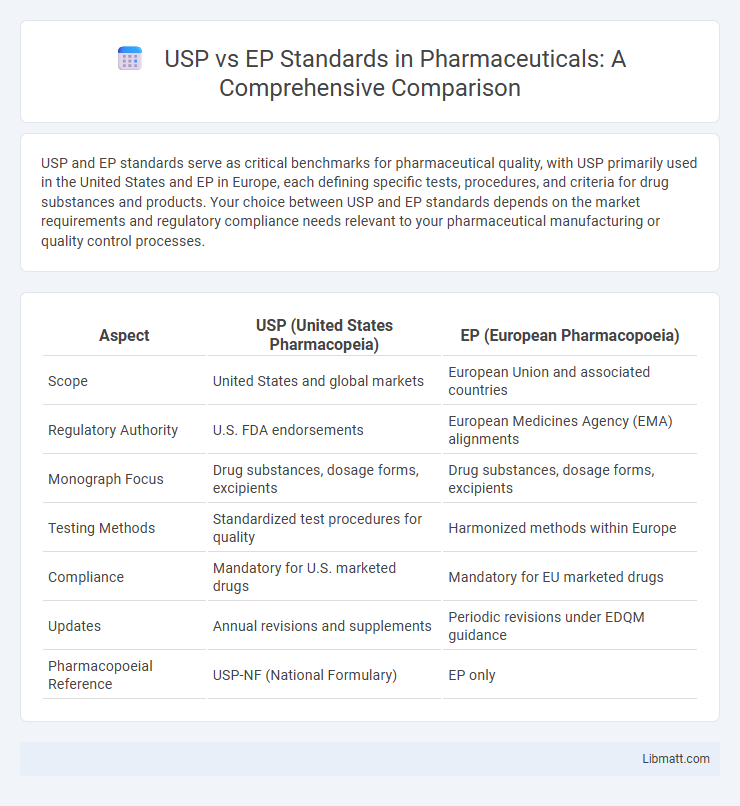
USP and EP standards serve as critical benchmarks for pharmaceutical quality, with USP primarily used in the United States and EP in Europe, each defining specific tests, procedures, and criteria for drug substances and products. Your choice between USP and EP standards depends on the market requirements and regulatory compliance needs relevant to your pharmaceutical manufacturing or quality control processes.
Table of Comparison
| Aspect | USP (United States Pharmacopeia) | EP (European Pharmacopoeia) |
|---|---|---|
| Scope | United States and global markets | European Union and associated countries |
| Regulatory Authority | U.S. FDA endorsements | European Medicines Agency (EMA) alignments |
| Monograph Focus | Drug substances, dosage forms, excipients | Drug substances, dosage forms, excipients |
| Testing Methods | Standardized test procedures for quality | Harmonized methods within Europe |
| Compliance | Mandatory for U.S. marketed drugs | Mandatory for EU marketed drugs |
| Updates | Annual revisions and supplements | Periodic revisions under EDQM guidance |
| Pharmacopoeial Reference | USP-NF (National Formulary) | EP only |
Introduction to USP and EP Standards
USP (United States Pharmacopeia) and EP (European Pharmacopoeia) are authoritative compendiums establishing quality standards for medicines, ensuring drug safety and efficacy. USP primarily serves the United States, providing detailed monographs, tests, and specifications for pharmaceuticals, while EP applies across Europe with harmonized standards for active ingredients, excipients, and dosage forms. Both pharmacopeias play a critical role in regulatory compliance, drug formulation, and quality control within their respective jurisdictions.
Overview of Pharmaceutical Quality Standards
USP (United States Pharmacopeia) and EP (European Pharmacopeia) are leading pharmaceutical quality standards ensuring drug safety, efficacy, and consistency. USP standards primarily apply in the United States, while EP standards cover Europe and several other countries, both providing detailed monographs, testing methods, and quality specifications for active ingredients and finished products. Your pharmaceutical products must comply with the relevant pharmacopeial guidelines to meet regulatory requirements and ensure global market acceptance.
Key Differences Between USP and EP
USP and EP standards differ primarily in geographic scope, with USP widely used in the United States and EP predominantly in Europe. USP emphasizes monographs tailored to the US pharmaceutical market, while EP covers broader European regulatory requirements and harmonizes with international guidelines. Your compliance strategy should consider these distinctions to meet regional regulatory expectations effectively.
Scope and Application of USP Standards
USP standards primarily apply to pharmaceuticals, dietary supplements, and food ingredients in the United States, ensuring product quality, purity, strength, and consistency. These standards are recognized by the U.S. Food and Drug Administration (FDA) and are legally enforceable for drug products marketed domestically. USP's comprehensive monographs, general chapters, and reference standards support manufacturers, regulators, and laboratories in quality control and regulatory compliance.
Scope and Application of EP Standards
European Pharmacopoeia (EP) standards cover medicinal substances, dosage forms, and pharmaceutical ingredients with a primary focus on Europe and countries that recognize EP compliance. The EP ensures uniform quality, safety, and efficacy requirements for medicines, pharmaceuticals, and excipients used within its jurisdiction. Your pharmaceutical products must meet EP standards when marketed in Europe to ensure regulatory approval and maintain consistent quality.
Testing Methods: USP vs EP
USP testing methods emphasize detailed pharmacopoeial monographs with specific analytical techniques tailored for the U.S. market, including a wide range of microbiological and chemical tests. EP testing methods provide comprehensive harmonized standards for the European market, employing advanced chromatographic and spectroscopic analyses to ensure drug quality and safety. Both standards prioritize accuracy and reproducibility but differ in procedural specifics and regional regulatory requirements.
Regulatory Acceptance and Compliance
USP (United States Pharmacopeia) and EP (European Pharmacopoeia) standards differ in regulatory acceptance due to jurisdiction-specific mandates, with USP primarily recognized in the United States and EP in European Union member states. Both standards are critical for pharmaceutical compliance, requiring manufacturers to adhere to the respective monographs for drug quality, safety, and efficacy in their target markets. Regulatory agencies such as the FDA and EMA enforce compliance by referencing USP and EP standards in drug approval and post-market surveillance processes.
Global Relevance and Harmonization Efforts
USP and EP standards play critical roles in ensuring pharmaceutical quality, with USP primarily dominant in the United States and EP widely recognized across Europe and other regions. Continuous harmonization efforts by the International Council for Harmonisation (ICH) aim to align USP and EP monographs and test methods to facilitate global regulatory compliance and streamline drug approval processes. This global relevance supports consistent pharmaceutical quality, safety, and efficacy, benefiting manufacturers and regulators worldwide.
Challenges in Switching Between USP and EP
Switching between USP (United States Pharmacopeia) and EP (European Pharmacopoeia) standards presents challenges such as differences in monograph requirements, testing methods, and acceptance criteria. Manufacturers must adapt to variations in analytical techniques, specifications, and compliance procedures, which can increase development time and costs. Regulatory consistency issues and the need for dual qualification of methods complicate harmonization efforts in global pharmaceutical markets.
Choosing the Right Standard for Your Product
Selecting the right pharmaceutical standard between USP (United States Pharmacopeia) and EP (European Pharmacopoeia) depends on your product's target market and regulatory requirements. USP standards are primarily recognized in the United States, ensuring compliance with FDA regulations, while EP standards cater to the European Union with guidelines aligned to EMA. Understanding the specific compendial requirements and testing protocols of your product's intended region will guide your choice for quality assurance and market approval.
USP vs EP standards Infographic
 libmatt.com
libmatt.com