Copper metallization offers superior electrical conductivity and better electromigration resistance compared to aluminum metallization, leading to enhanced performance and reliability in integrated circuits. Your choice depends on factors such as cost, fabrication complexity, and specific application requirements, with copper being preferred for high-performance devices and aluminum for cost-effective, simpler processes.
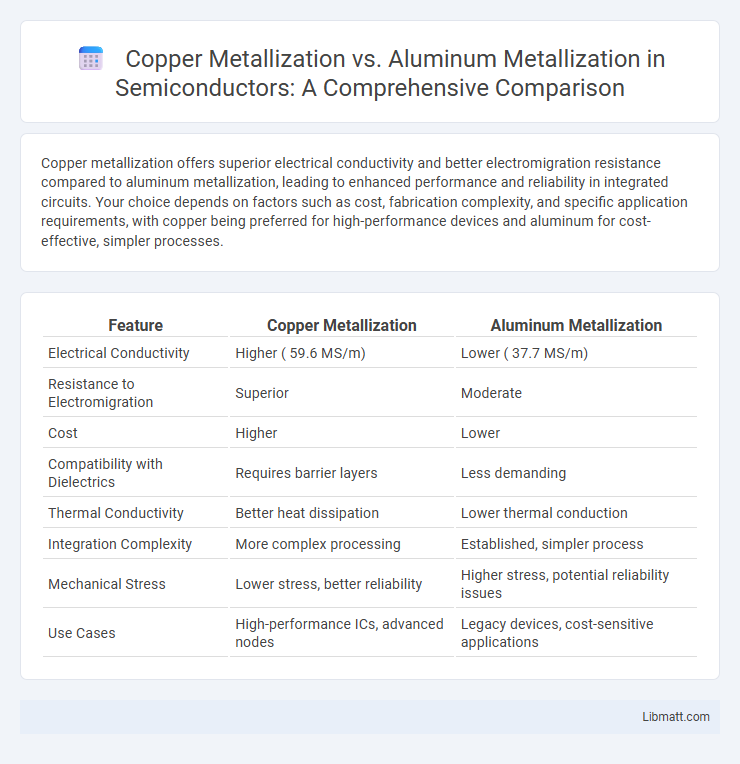
Table of Comparison
| Feature | Copper Metallization | Aluminum Metallization |
|---|---|---|
| Electrical Conductivity | Higher ( 59.6 MS/m) | Lower ( 37.7 MS/m) |
| Resistance to Electromigration | Superior | Moderate |
| Cost | Higher | Lower |
| Compatibility with Dielectrics | Requires barrier layers | Less demanding |
| Thermal Conductivity | Better heat dissipation | Lower thermal conduction |
| Integration Complexity | More complex processing | Established, simpler process |
| Mechanical Stress | Lower stress, better reliability | Higher stress, potential reliability issues |
| Use Cases | High-performance ICs, advanced nodes | Legacy devices, cost-sensitive applications |
Introduction to Metallization in Semiconductor Devices
Metallization in semiconductor devices involves depositing metal layers to form interconnections and contacts essential for electrical functionality. Copper metallization offers superior electrical conductivity and electromigration resistance compared to aluminum, enabling higher performance and reliability in integrated circuits. Aluminum metallization, historically dominant due to ease of deposition and cost-effectiveness, exhibits higher resistivity and limited scalability for advanced technology nodes.
Overview of Copper and Aluminum Metallization
Copper metallization offers superior electrical conductivity and enhanced electromigration resistance compared to aluminum metallization, making it ideal for advanced semiconductor devices. Aluminum metallization remains widely used due to its lower cost, easier integration, and proven reliability in traditional microelectronic fabrication. The choice between copper and aluminum metallization directly impacts device performance, thermal management, and manufacturing complexity in integrated circuit production.
Historical Evolution of Metallization Materials
Copper metallization emerged as a superior alternative to aluminum metallization due to copper's lower resistivity and better electromigration resistance, leading to enhanced performance in semiconductor devices. Aluminum was predominant from the 1960s until the late 1990s when scaling limitations and increased current densities highlighted its inefficiencies. Your choice of metallization material directly impacts device reliability and speed, with copper now favored in advanced integrated circuits for its improved electrical and thermal conductivity.
Electrical Conductivity: Copper vs Aluminum
Copper metallization offers significantly higher electrical conductivity, approximately 59.6 x 10^6 S/m, compared to aluminum's 37.8 x 10^6 S/m, enabling more efficient current flow and reduced resistive losses in semiconductor devices. This superior conductivity of copper results in lower power consumption and improved performance in integrated circuits, especially in high-speed microprocessors. Despite aluminum's cost-effectiveness and ease of fabrication, copper's enhanced conductivity makes it the preferred choice for advanced metallization layers in modern electronics.
Electromigration Resistance Comparison
Copper metallization demonstrates significantly higher electromigration resistance compared to aluminum metallization due to its lower atomic diffusivity and stronger grain boundary cohesion. This improved resistance enables copper interconnects to sustain higher current densities and extended operational lifetimes in integrated circuits. As a result, copper-based interconnects are preferred in advanced semiconductor manufacturing for enhanced reliability and performance.
Manufacturing Process Differences
Copper metallization involves more complex manufacturing steps than aluminum metallization due to copper's tendency to diffuse into silicon, requiring the application of diffusion barrier layers such as tantalum or tantalum nitride. Unlike aluminum, copper cannot be patterned using traditional etching and instead uses a damascene process, where trenches are etched into the dielectric, filled with copper, and then polished using chemical mechanical planarization (CMP). Your choice between copper and aluminum metallization impacts fabrication costs, process complexity, and device reliability.
Cost Analysis of Copper and Aluminum Metallization
Copper metallization typically involves higher initial costs due to expensive raw materials and more complex deposition processes, but it offers superior electrical conductivity and reliability that can reduce overall system costs in high-performance applications. Aluminum metallization is generally more cost-effective upfront because of lower material prices and simpler fabrication methods, making it suitable for budget-sensitive projects with moderate performance requirements. Your choice between copper and aluminum metallization should weigh the trade-off between initial investment and long-term efficiency and durability benefits in semiconductor manufacturing.
Reliability and Longevity Factors
Copper metallization offers superior reliability and longevity compared to aluminum due to its lower electrical resistivity and higher electromigration resistance, which reduces the risk of circuit failures over time. The higher thermal conductivity of copper enhances heat dissipation, improving device stability under high-stress conditions. Aluminum's susceptibility to oxidation and hillock formation can compromise circuit integrity, making copper the preferred choice for advanced semiconductor interconnects requiring enhanced performance and durability.
Environmental and Safety Considerations
Copper metallization offers significant environmental advantages over aluminum due to its superior electrical efficiency, which reduces energy consumption in semiconductor devices. Copper's lower resistivity decreases heat generation, mitigating thermal hazards and enhancing device safety. While copper requires careful handling to prevent contamination and corrosion, its recyclability and reduced material waste contribute to a more sustainable manufacturing process compared to aluminum.
Future Trends in Semiconductor Metallization
Copper metallization offers superior electrical conductivity and electromigration resistance compared to aluminum, making it the preferred choice for advanced semiconductor devices. Emerging trends emphasize integrating copper with low-k dielectrics to reduce capacitance and improve device performance at smaller nodes. Your semiconductor designs will benefit from ongoing innovations in copper barrier layers and deposition techniques to meet future scaling and reliability demands.
Copper Metallization vs Aluminum Metallization Infographic
 libmatt.com
libmatt.com