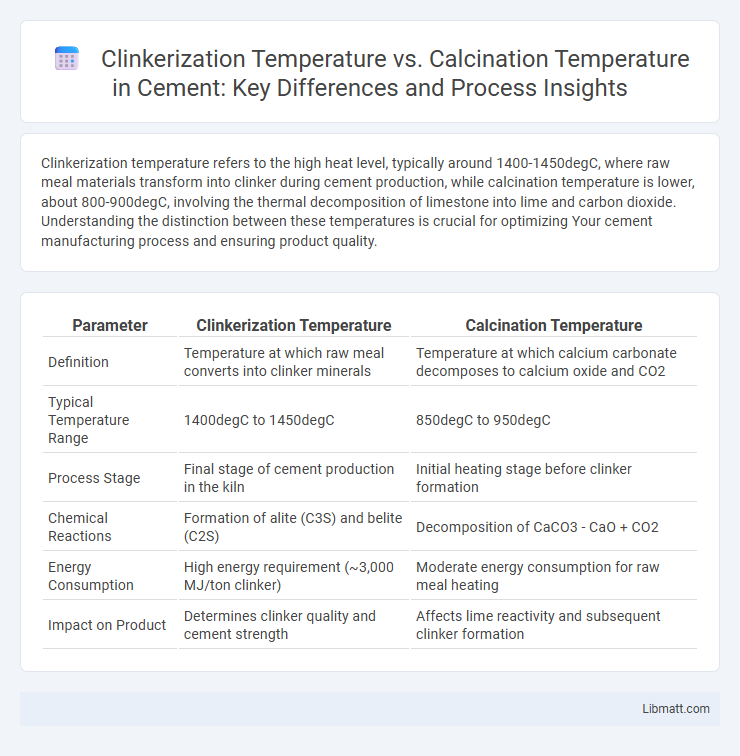
Clinkerization temperature refers to the high heat level, typically around 1400-1450degC, where raw meal materials transform into clinker during cement production, while calcination temperature is lower, about 800-900degC, involving the thermal decomposition of limestone into lime and carbon dioxide. Understanding the distinction between these temperatures is crucial for optimizing Your cement manufacturing process and ensuring product quality.
Table of Comparison
| Parameter | Clinkerization Temperature | Calcination Temperature |
|---|---|---|
| Definition | Temperature at which raw meal converts into clinker minerals | Temperature at which calcium carbonate decomposes to calcium oxide and CO2 |
| Typical Temperature Range | 1400degC to 1450degC | 850degC to 950degC |
| Process Stage | Final stage of cement production in the kiln | Initial heating stage before clinker formation |
| Chemical Reactions | Formation of alite (C3S) and belite (C2S) | Decomposition of CaCO3 - CaO + CO2 |
| Energy Consumption | High energy requirement (~3,000 MJ/ton clinker) | Moderate energy consumption for raw meal heating |
| Impact on Product | Determines clinker quality and cement strength | Affects lime reactivity and subsequent clinker formation |
Understanding Clinkerization and Calcination Processes
Clinkerization temperature typically ranges from 1,400degC to 1,450degC, essential for forming clinker nodules through complex chemical reactions in the kiln. Calcination temperature, usually around 900degC, drives off carbon dioxide from limestone, converting it to lime and marking the initial stage of cement production. Understanding these temperature differences helps optimize Your cement quality and energy efficiency during manufacturing.
Defining Clinkerization Temperature
Clinkerization temperature refers to the peak heat level at which raw materials in cement production transform into clinker through partial melting and chemical reactions, typically ranging from 1400degC to 1500degC. This temperature is crucial because it facilitates the formation of essential mineral phases like alite and belite, directly impacting cement quality and strength. Unlike calcination temperature, which usually occurs around 900degC and involves decomposing limestone into lime and carbon dioxide, clinkerization demands significantly higher thermal conditions to achieve clinker fusion and nodulation.
What Is Calcination Temperature?
Calcination temperature refers to the specific heat level at which raw materials, typically limestone or other carbonates, are heated to induce thermal decomposition, driving off carbon dioxide and forming clinker minerals. This temperature usually ranges between 850degC and 1000degC, distinct from clinkerization temperature, which is higher, around 1400degC to 1450degC, where clinker minerals undergo sintering and fusion. Precise control of calcination temperature is crucial for optimizing the chemical reactions that form key cement compounds such as lime and silicates before clinker production.
Chemical Reactions in Clinkerization vs Calcination
Clinkerization involves complex high-temperature chemical reactions where raw meal components such as calcium carbonate, silica, alumina, and iron oxide react to form clinker minerals like alite and belite through processes including decarbonation, silicate formation, and phase transformations above 1300degC. Calcination occurs at lower temperatures, typically around 900degC, focusing primarily on the thermal decomposition of calcium carbonate into calcium oxide and carbon dioxide, without the formation of clinker minerals. The distinct temperature ranges directly influence the progression and type of chemical reactions, with calcination serving as a precursor step to the more intensive mineral synthesis during clinkerization.
Key Temperature Ranges in Cement Manufacturing
Clinkerization temperature typically ranges between 1,400degC and 1,450degC, essential for the formation of clinker nodules in cement manufacturing. Calcination temperature occurs earlier, around 750degC to 900degC, where calcium carbonate decomposes into lime and carbon dioxide, initiating the cement production process. Your understanding of these distinct temperature ranges is crucial for optimizing cement kiln performance and enhancing product quality.
Impact of Temperature on Product Quality
Clinkerization temperature, typically ranging between 1400degC to 1500degC, is crucial for forming high-quality clinker minerals that determine cement strength and durability, whereas calcination temperature, usually around 900degC to 1000degC, primarily drives the decomposition of raw materials but does not develop the final clinker phases. Higher clinkerization temperatures ensure complete sintering and optimal mineral formation, directly impacting the mechanical properties and long-term performance of cement. Understanding this temperature distinction helps optimize your production process to achieve superior product quality and reduce energy consumption.
Energy Consumption at Different Temperatures
Clinkerization temperature, typically ranging from 1400degC to 1500degC, requires significantly higher energy consumption compared to calcination temperature, which is generally around 900degC to 1000degC. The elevated temperature during clinkerization leads to greater fuel usage and CO2 emissions due to the intense chemical reactions taking place, such as the formation of alite and belite phases. Optimizing calcination processes can reduce overall energy demand by partially decomposing raw materials before clinkerization, thereby enhancing thermal efficiency in cement production.
Influence of Raw Materials on Temperature Requirements
Raw materials with high silica and alumina content typically increase the clinkerization temperature due to their refractory nature, while calcination temperatures are primarily influenced by the decomposition characteristics of limestone and clay. Variations in mineral composition, such as high iron oxide or magnesium content, can modify the energy required, as these elements affect phase formation and sintering behavior during clinkerization. Optimization of raw material composition directly impacts thermal efficiency, reducing fuel consumption and emissions in both calcination and clinkerization processes.
Operational Challenges in Maintaining Optimal Temperatures
Maintaining optimal clinkerization temperature between 1400degC and 1500degC is crucial to ensure complete mineral formation, while calcination temperature typically ranges from 900degC to 1000degC to effectively decompose limestone into lime and carbon dioxide. Operational challenges arise due to the high energy demands and fuel variability, leading to temperature fluctuations that can cause incomplete reactions or equipment damage. You must implement precise temperature control systems and real-time monitoring to stabilize the process and prevent inefficiencies or product quality issues.
Innovations for Temperature Optimization in Cement Production
Innovations in temperature optimization for clinkerization and calcination processes focus on advanced sensor technologies and AI-driven control systems to precisely monitor and adjust thermal profiles, reducing energy consumption and CO2 emissions. Developments in alternative fuel integration and high-efficiency rotary kilns enable stable clinkerization at lower temperatures without compromising cement quality. Enhanced thermal modeling and real-time data analytics improve the synchronization between calcination and clinkerization stages, maximizing process efficiency and sustainability in cement production.
Clinkerization Temperature vs Calcination Temperature Infographic
 libmatt.com
libmatt.com