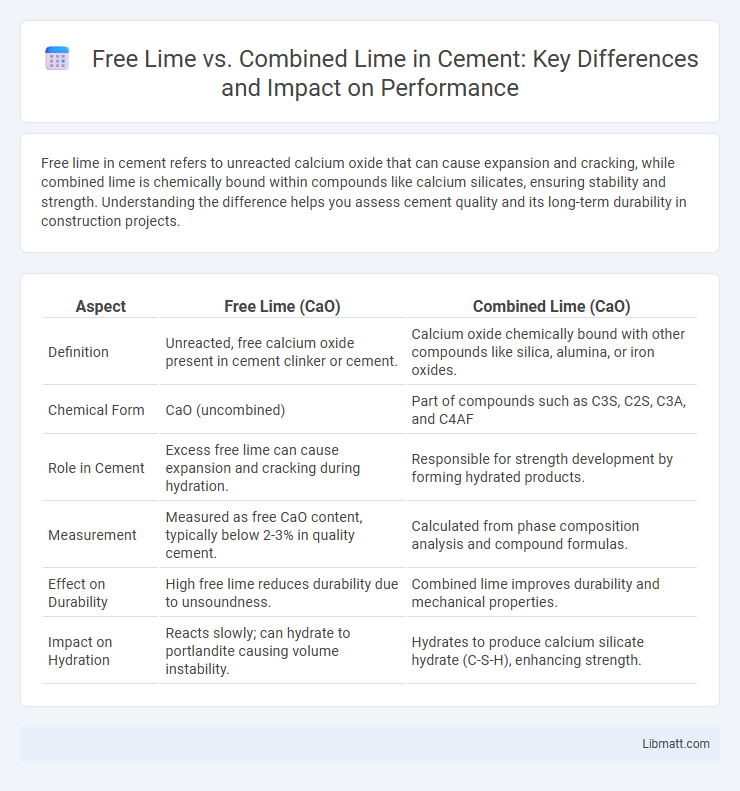
Free lime in cement refers to unreacted calcium oxide that can cause expansion and cracking, while combined lime is chemically bound within compounds like calcium silicates, ensuring stability and strength. Understanding the difference helps you assess cement quality and its long-term durability in construction projects.
Table of Comparison
| Aspect | Free Lime (CaO) | Combined Lime (CaO) |
|---|---|---|
| Definition | Unreacted, free calcium oxide present in cement clinker or cement. | Calcium oxide chemically bound with other compounds like silica, alumina, or iron oxides. |
| Chemical Form | CaO (uncombined) | Part of compounds such as C3S, C2S, C3A, and C4AF |
| Role in Cement | Excess free lime can cause expansion and cracking during hydration. | Responsible for strength development by forming hydrated products. |
| Measurement | Measured as free CaO content, typically below 2-3% in quality cement. | Calculated from phase composition analysis and compound formulas. |
| Effect on Durability | High free lime reduces durability due to unsoundness. | Combined lime improves durability and mechanical properties. |
| Impact on Hydration | Reacts slowly; can hydrate to portlandite causing volume instability. | Hydrates to produce calcium silicate hydrate (C-S-H), enhancing strength. |
Introduction to Lime in Soil Science
Free lime in soil refers to the amount of calcium oxide (CaO) or magnesium oxide (MgO) present in an uncombined form, which can affect soil alkalinity and nutrient availability. Combined lime consists of calcium or magnesium bound within carbonate minerals, primarily calcium carbonate (CaCO3), influencing soil pH more gradually through dissolution processes. Understanding the distinction between free lime and combined lime is essential for effective soil amendment, as free lime reacts quickly to neutralize acidity while combined lime provides a more sustained pH adjustment.
Definition of Free Lime
Free lime refers to uncombined calcium oxide or hydroxide present in lime products that has not reacted with other elements during processing. In contrast, combined lime consists of calcium compounds chemically bonded with silica, alumina, or iron oxides, forming stable minerals like calcium silicates. Understanding the free lime content is crucial for Your application, as it directly influences the reactivity, setting time, and durability of lime-based materials.
Definition of Combined Lime
Combined lime is a blend of free lime (calcium oxide) and hydrated lime (calcium hydroxide) used in construction and environmental applications for soil stabilization and water treatment. The combination enhances the material's reactivity and durability compared to using free lime alone. This blend optimizes pozzolanic reactions, improving long-term strength and reducing permeability in treated soils.
Sources of Lime in Soil
Free lime in soil primarily originates from calcium carbonate minerals like limestone and chalk, which remain unaltered and dissolve slowly, influencing soil pH and structure. Combined lime forms when free lime reacts with soil components such as clay or organic matter, creating bound compounds like calcium silicate or calcium humate that affect nutrient availability. Understanding these sources helps you manage soil fertility more effectively by choosing the right lime type for your soil conditions.
Chemical Forms of Lime
Free lime primarily consists of calcium oxide (CaO) and calcium hydroxide (Ca(OH)2), which are uncombined forms available in quicklime and hydrated lime. Combined lime refers to calcium bound in compounds such as calcium carbonate (CaCO3), calcium silicates, and aluminates found in natural limestone and dolomitic lime. The chemical reactivity and properties of lime depend on these forms, with free lime reacting rapidly in cementitious processes, while combined lime provides long-term strength through gradual transformation.
Methods of Identifying Free and Combined Lime
Free lime and combined lime are primarily identified through chemical analysis techniques such as ethylene blue staining and X-ray diffraction (XRD). Ethylene blue staining reacts specifically with free lime, turning it blue, which helps distinguish it from combined lime that remains uncolored. XRD provides a detailed mineralogical profile by detecting crystalline phases, enabling precise quantification of both free and combined lime in cementitious materials.
Importance in Soil Fertility
Free lime and combined lime play crucial roles in soil fertility by regulating pH levels and improving nutrient availability. Free lime, primarily calcium carbonate, neutralizes soil acidity rapidly, promoting microbial activity and enhancing nutrient uptake by plants. Combined lime, containing magnesium as well as calcium, addresses both pH correction and magnesium deficiency, contributing to balanced soil chemistry and optimal crop growth.
Effects on Soil pH and Structure
Free lime primarily raises soil pH more rapidly by neutralizing acidity, improving nutrient availability for plants, while combined lime releases calcium more slowly, offering a gradual pH adjustment. Soil structure benefits from free lime through enhanced aggregation and reduced compaction, promoting better aeration and water infiltration; combined lime contributes to long-term soil stability by supplying calcium over time. Understanding these differences helps you select the appropriate lime type for effective soil pH management and improved soil health.
Agricultural Implications of Free vs Combined Lime
Free lime primarily consists of calcium oxide and has a strong neutralizing effect on acidic soils, rapidly increasing soil pH and improving nutrient availability for crops. Combined lime contains calcium carbonate and magnesium carbonate, providing a slower but longer-lasting pH adjustment while supplying essential calcium and magnesium nutrients beneficial for soil structure. Understanding the differences helps you choose the appropriate lime type to optimize soil health, enhance crop yield, and manage nutrient balance effectively.
Summary: Choosing the Right Lime Form
Free lime and combined lime play distinct roles in soil amendment and water treatment, with free lime being calcium oxide/hydroxide readily available for neutralization and combined lime existing as chemically bound calcium compounds requiring conversion. Understanding the differences in reactivity and application efficiency helps optimize soil pH management and industrial processes. Your choice depends on the immediate lime availability needed and long-term soil conditioning goals.
Free Lime vs Combined Lime Infographic
 libmatt.com
libmatt.com