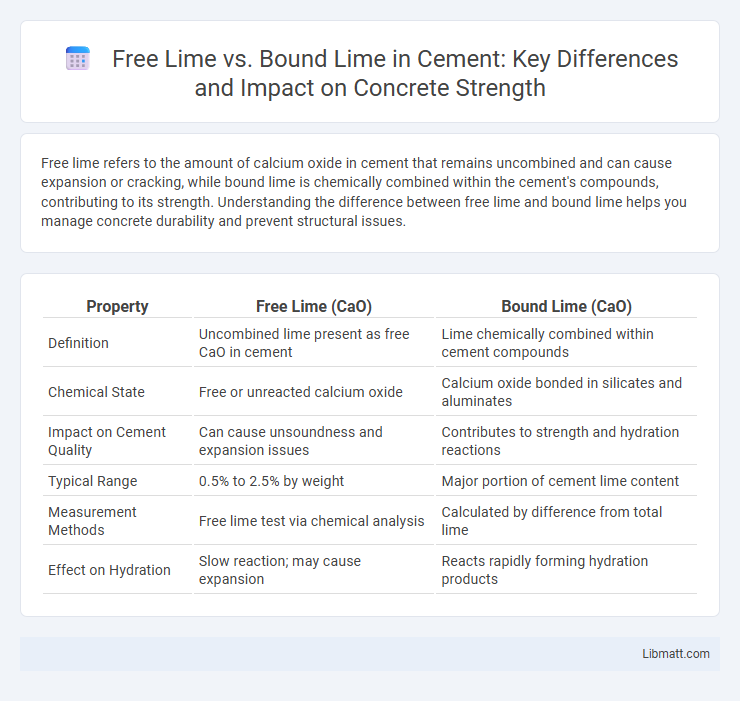
Free lime refers to the amount of calcium oxide in cement that remains uncombined and can cause expansion or cracking, while bound lime is chemically combined within the cement's compounds, contributing to its strength. Understanding the difference between free lime and bound lime helps you manage concrete durability and prevent structural issues.
Table of Comparison
| Property | Free Lime (CaO) | Bound Lime (CaO) |
|---|---|---|
| Definition | Uncombined lime present as free CaO in cement | Lime chemically combined within cement compounds |
| Chemical State | Free or unreacted calcium oxide | Calcium oxide bonded in silicates and aluminates |
| Impact on Cement Quality | Can cause unsoundness and expansion issues | Contributes to strength and hydration reactions |
| Typical Range | 0.5% to 2.5% by weight | Major portion of cement lime content |
| Measurement Methods | Free lime test via chemical analysis | Calculated by difference from total lime |
| Effect on Hydration | Slow reaction; may cause expansion | Reacts rapidly forming hydration products |
Introduction to Free Lime and Bound Lime
Free lime refers to the calcium oxide or hydroxide present in cement or lime products that has not reacted chemically and remains available to react with water or other substances. Bound lime, on the other hand, is chemically combined within compounds like calcium silicates or aluminates, forming stable structures that contribute to the strength and durability of materials. Understanding the distinction between free lime and bound lime is crucial for quality control in cement manufacturing and assessing hydration processes.
Understanding Lime Chemistry
Free lime refers to calcium oxide (CaO) or calcium hydroxide (Ca(OH)2) present in lime products that has not reacted with other components, influencing setting time and durability in construction materials. Bound lime is chemically combined with silica, alumina, or other compounds forming calcium silicates or aluminates, contributing to strength development in mortars and concretes. Understanding the proportion of free lime versus bound lime is crucial for optimizing lime's performance in building applications and preventing issues like excessive expansion or poor bonding.
Definition of Free Lime
Free lime refers to calcium oxide (CaO) present in lime that has not reacted with other components and remains chemically available. Unlike bound lime, free lime can cause unsoundness in construction materials due to its ability to hydrate and expand over time. Understanding the proportion of free lime in your lime source is crucial for predicting durability and performance in cement and mortar applications.
Definition of Bound Lime
Bound lime refers to the calcium compounds in cement or lime mixtures that are chemically combined with silicates or aluminates, forming stable compounds such as calcium silicate hydrates and calcium aluminate hydrates. Unlike free lime, which remains as unreacted calcium oxide or hydroxide, bound lime contributes to the strength and durability of building materials by enhancing hydration and pozzolanic reactions. The presence of bound lime is crucial for improving the mechanical properties and long-term performance of concrete and mortar.
Sources of Free Lime in Materials
Free lime primarily originates from calcium oxide (CaO) present in lime-based materials such as quicklime and certain types of cement. It often forms during the incomplete hydration or calcination processes, where calcium carbonate decomposes but does not fully react. Common sources of free lime in construction materials include unreacted lime particles in portland cement, lime putty, and hydrated lime products used in mortar and plaster.
Formation and Occurrence of Bound Lime
Bound lime forms when free lime reacts chemically with silica, alumina, and iron oxides in cement clinker during the cooling phase, creating compounds like belite (C2S), alite (C3S), and aluminate phases. This chemically combined lime is integral to cement's strength development and durability, distinguishing it from free lime, which remains unreacted and can cause detrimental expansion. Bound lime stabilizes the cement matrix by reducing free lime content through its incorporation into these complex mineral phases.
Key Differences: Free Lime vs Bound Lime
Free lime refers to calcium oxide (CaO) or calcium hydroxide (Ca(OH)2) present in a material that is not chemically combined and remains reactive, while bound lime is chemically combined with other compounds such as silica, alumina, or iron oxides, forming stable minerals. The key difference lies in their chemical reactivity and impact on setting and durability: free lime can cause expansion and cracking if not properly controlled, whereas bound lime contributes to the strength and hardness of materials like cement or soil stabilization. Understanding these differences helps optimize your processes in construction, masonry, or soil treatment applications.
Role of Lime States in Industrial Applications
Free lime (CaO) plays a critical role in industries such as steel manufacturing and construction by providing rapid reactivity and high alkalinity for processes like slag formation and cement setting. Bound lime, found in compounds like calcium hydroxide or calcium carbonate, offers controlled release and stability, essential for applications in water treatment and soil stabilization. Understanding the balance between free and bound lime states enhances efficiency in chemical reactions, durability in construction materials, and environmental treatment outcomes.
Testing and Determination Methods
Testing free lime involves chemical titration methods such as the ethylenediaminetetraacetic acid (EDTA) titration and complexometric analysis, which measure the uncombined calcium oxide in cement. Bound lime is typically determined through thermal analysis techniques, including thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), to assess the calcium chemically combined with other compounds. X-ray diffraction (XRD) and scanning electron microscopy (SEM) are also utilized to distinguish free and bound lime phases by analyzing mineralogical characteristics.
Implications for Construction and Material Performance
Free lime in construction materials can cause expansion and cracking due to its delayed hydration, compromising structural integrity and durability. Bound lime, chemically combined within compounds such as calcium silicates and aluminates, contributes to material strength and stability by improving cementitious properties. Understanding the balance between free and bound lime content is critical for optimizing concrete performance and preventing long-term damage in building applications.
Free lime vs Bound lime Infographic
 libmatt.com
libmatt.com