Gypsum and anhydrite are both calcium sulfate minerals, but gypsum contains water molecules making it softer and more suitable for applications like drywall and plaster, while anhydrite is harder and used mainly in industrial processes requiring a moisture-free environment. Understanding the differences helps you select the right material for construction or manufacturing needs.
Table of Comparison
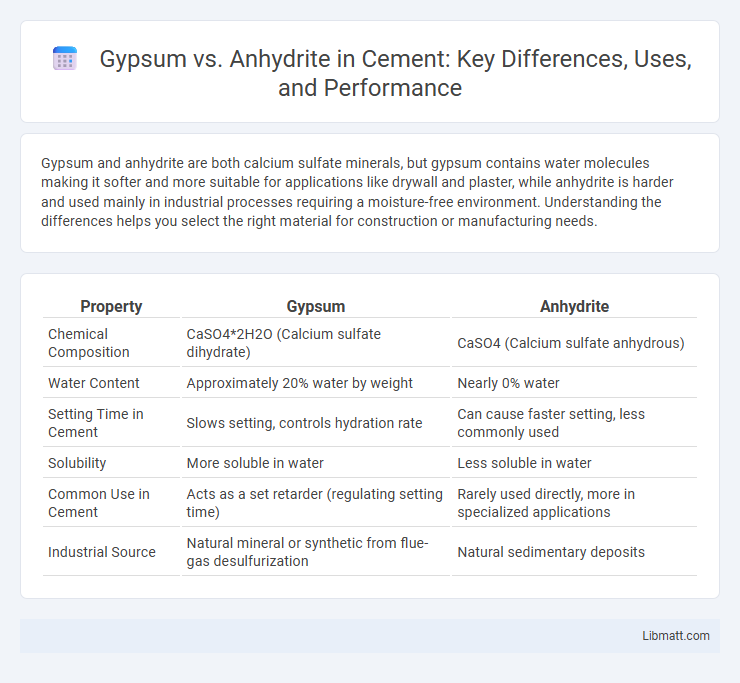
| Property | Gypsum | Anhydrite |
|---|---|---|
| Chemical Composition | CaSO4*2H2O (Calcium sulfate dihydrate) | CaSO4 (Calcium sulfate anhydrous) |
| Water Content | Approximately 20% water by weight | Nearly 0% water |
| Setting Time in Cement | Slows setting, controls hydration rate | Can cause faster setting, less commonly used |
| Solubility | More soluble in water | Less soluble in water |
| Common Use in Cement | Acts as a set retarder (regulating setting time) | Rarely used directly, more in specialized applications |
| Industrial Source | Natural mineral or synthetic from flue-gas desulfurization | Natural sedimentary deposits |
Understanding Gypsum and Anhydrite: An Introduction
Gypsum and anhydrite are both calcium sulfate minerals commonly used in construction and agriculture, with gypsum containing water molecules (CaSO4*2H2O) and anhydrite lacking them (CaSO4). Understanding the chemical and physical differences between gypsum and anhydrite is crucial for selecting the right material for your project, as gypsum is softer and more soluble, while anhydrite is harder and less reactive. Knowledge of these properties helps optimize material performance in applications like drywall manufacturing, soil conditioning, and cement production.
Chemical Composition Differences
Gypsum consists primarily of calcium sulfate dihydrate (CaSO4*2H2O), which contains two crystal water molecules, while anhydrite is calcium sulfate without water (CaSO4). This difference in hydration results in gypsum being softer and more soluble than anhydrite, which is denser and harder due to the absence of water molecules. Understanding these chemical composition variations helps you select the appropriate material for applications like construction, agriculture, or industrial processes.
Physical Properties Comparison
Gypsum has a Mohs hardness of 2, making it soft enough to be scratched by a fingernail, while anhydrite is harder, with a Mohs hardness around 3 to 3.5. Gypsum typically exhibits a white to translucent appearance with a monoclinic crystal system, whereas anhydrite is more often gray, blue, or reddish and crystallizes in the orthorhombic system. The specific gravity of gypsum ranges from 2.31 to 2.33, lower than anhydrite's specific gravity of 2.93 to 2.98, reflecting differences in their water content and density.
Formation and Geological Occurrence
Gypsum forms through the evaporation of seawater in sedimentary basins, commonly found in evaporite deposits alongside halite and other sulfate minerals. Anhydrite, composed of anhydrous calcium sulfate, typically forms from the dehydration of gypsum during burial or in arid depositional environments with limited water activity. Both minerals frequently occur in sedimentary sequences from marine evaporitic settings, but anhydrite is more prevalent at greater depths and higher temperatures due to thermal and diagenetic transformation processes.
Industrial and Construction Uses
Gypsum is widely used in the construction industry for producing plaster, drywall, and cement due to its excellent fire resistance and easy workability, while anhydrite serves as a drying agent and an additive in cement to control setting time. In industrial applications, gypsum's solubility supports soil conditioning and as a filler in paints and paper, whereas anhydrite is favored for its low water content in manufacturing sulfuric acid and as a calcium source in agricultural lime. Your choice between gypsum and anhydrite impacts material performance, especially in setting time control and moisture management in construction projects.
Processing and Refining Methods
Gypsum undergoes calcination to remove water content, producing plaster of Paris, while anhydrite requires less processing due to its naturally dehydrated state. Refining gypsum involves grinding and washing to eliminate impurities, whereas anhydrite is typically ground and sometimes blended with additives to enhance its performance in cement and plaster applications. Your choice between gypsum and anhydrite depends on the desired texture and setting time, informed by their distinct refining processes.
Advantages of Gypsum Over Anhydrite
Gypsum offers superior water retention and ease of handling compared to anhydrite, making it ideal for agricultural and construction applications. Its natural presence of chemically combined water aids in soil conditioning and faster curing times in plaster and drywall usage. When choosing materials for your project, gypsum's enhanced moisture regulation benefits can improve durability and workability.
Challenges and Limitations of Anhydrite
Anhydrite presents several challenges and limitations compared to gypsum in construction and agricultural applications. Its low solubility and slower hydration rate can hinder soil amendment and industrial processes, while its tendency to absorb moisture and convert to gypsum causes volume instability and structural concerns. Understanding these factors helps you choose the appropriate material for your specific project requirements.
Environmental Impact and Sustainability
Gypsum has a lower environmental impact compared to anhydrite due to its abundant availability and ease of recycling in construction applications, reducing resource depletion. Anhydrite mining often requires more energy-intensive processing, increasing carbon emissions and ecological disturbance. Understanding these differences helps you choose more sustainable materials for environmentally responsible building projects.
Choosing the Right Material: Gypsum or Anhydrite?
Choosing between gypsum and anhydrite depends on your project's moisture requirements and setting time. Gypsum contains water of crystallization, allowing for faster setting and easier workability in damp environments, while anhydrite, being an anhydrous form of calcium sulfate, offers slower setting times and greater hardness when dry. Your decision should factor in whether quick drying or increased durability in low-moisture conditions is more critical to your construction or finishing needs.
Gypsum vs Anhydrite Infographic
 libmatt.com
libmatt.com