Latent heat is the energy absorbed or released during a phase change without changing temperature, while sensible heat causes a temperature change in a substance without a phase transition. Understanding the difference helps you predict how substances behave under heat transfer in various processes.
Table of Comparison
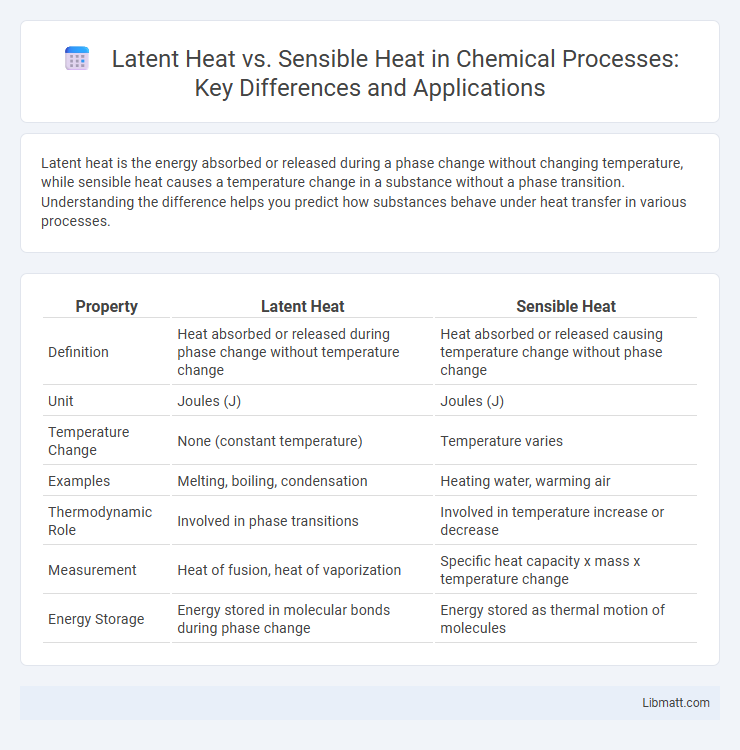
| Property | Latent Heat | Sensible Heat |
|---|---|---|
| Definition | Heat absorbed or released during phase change without temperature change | Heat absorbed or released causing temperature change without phase change |
| Unit | Joules (J) | Joules (J) |
| Temperature Change | None (constant temperature) | Temperature varies |
| Examples | Melting, boiling, condensation | Heating water, warming air |
| Thermodynamic Role | Involved in phase transitions | Involved in temperature increase or decrease |
| Measurement | Heat of fusion, heat of vaporization | Specific heat capacity x mass x temperature change |
| Energy Storage | Energy stored in molecular bonds during phase change | Energy stored as thermal motion of molecules |
Introduction to Heat Transfer
Heat transfer involves the movement of energy due to temperature differences, categorized into latent heat and sensible heat. Sensible heat causes a change in temperature of a substance without altering its phase, measurable by a thermometer. Latent heat refers to the energy absorbed or released during a phase change, such as melting or vaporization, without a temperature change.
Defining Latent Heat
Latent heat refers to the energy absorbed or released by a substance during a phase change without a temperature change, such as melting, boiling, or condensation. This energy is used to alter the molecular structure, breaking or forming intermolecular bonds, rather than raising the temperature. In contrast, sensible heat causes a temperature change in a substance without changing its phase.
Understanding Sensible Heat
Sensible heat refers to the heat exchanged by a substance resulting in a temperature change that can be measured with a thermometer, without altering the phase of the substance. It plays a crucial role in processes like heating or cooling air, where the temperature shifts but no phase change, such as melting or evaporation, occurs. Understanding sensible heat helps you accurately assess energy transfer in HVAC systems, meteorology, and food processing.
Key Differences Between Latent and Sensible Heat
Latent heat refers to the energy absorbed or released during a phase change of a substance without a change in temperature, whereas sensible heat involves energy transfer that causes a measurable temperature change. Your understanding of these key differences is critical in thermodynamics and HVAC systems, where latent heat affects humidity control and sensible heat influences temperature regulation. Sensible heat is detectable by a thermometer, but latent heat remains hidden until the phase change completes.
Measurement Techniques for Latent and Sensible Heat
Measurement techniques for latent heat primarily involve calorimetry using evaporimeters or hygrometers to quantify moisture exchange during phase changes, while sensible heat is measured by temperature changes detected with thermocouples or resistance temperature detectors (RTDs) in controlled environments. Instruments like Bowen ratio systems apply aerodynamic methods to distinguish between latent and sensible heat fluxes by measuring gradients of temperature and humidity above surfaces. Satellite remote sensing technologies use thermal infrared sensors combined with radiative transfer models to estimate surface sensible heat flux and latent heat through evapotranspiration calculations.
Real-World Examples of Latent Heat
Latent heat plays a crucial role in processes such as the melting of ice, where energy is absorbed without a temperature change, and the evaporation of water, which cools your skin by removing heat during sweating. This energy exchange is vital in weather phenomena like cloud formation and rainfall, where water vapor condenses releasing latent heat that powers storm systems. Understanding latent heat helps optimize heating and cooling systems by managing energy involved in phase changes rather than temperature shifts.
Practical Applications of Sensible Heat
Sensible heat plays a crucial role in HVAC systems by controlling indoor temperature through the heating and cooling of air. It is essential in cooking processes, where temperature changes without phase change determine cooking times and food texture. Industrial drying and heating applications rely on sensible heat to efficiently raise temperatures without altering material states.
Importance in HVAC and Meteorology
Latent heat plays a crucial role in HVAC systems by influencing moisture control and energy efficiency during phase changes like evaporation and condensation, directly impacting indoor air quality and comfort. Sensible heat is vital for regulating temperature through direct heat transfer, enabling precise climate control in buildings and accurate temperature forecasting in meteorology. Understanding the balance between latent and sensible heat improves HVAC design and enhances weather prediction models by accurately representing heat and moisture fluxes in the atmosphere.
The Role of Latent and Sensible Heat in Phase Changes
Latent heat plays a crucial role in phase changes by facilitating the energy exchange required to alter a substance's state without changing its temperature, such as melting or vaporization. Sensible heat, on the other hand, changes the temperature of a substance without causing a phase change, affecting the thermal sensation you experience. Understanding the interplay between latent and sensible heat is essential for efficiently managing energy in processes like heating, cooling, and weather phenomena.
Summary: Choosing the Right Concept for Your Needs
Latent heat involves the energy absorbed or released during phase changes without temperature change, while sensible heat affects the temperature of a substance without altering its phase. Understanding the difference is crucial for applications in HVAC systems, meteorology, and material science to optimize energy efficiency and process control. Your choice between latent and sensible heat depends on whether temperature change or phase transition is the primary concern in your specific thermal management scenario.
Latent heat vs sensible heat Infographic
 libmatt.com
libmatt.com