Evaporation removes a liquid by converting it into vapor, concentrating the remaining solution, while crystallization forms solid crystals from a supersaturated liquid as it cools or evaporates. Your understanding of these complementary processes helps in applications such as salt extraction, purification, and chemical manufacturing.
Table of Comparison
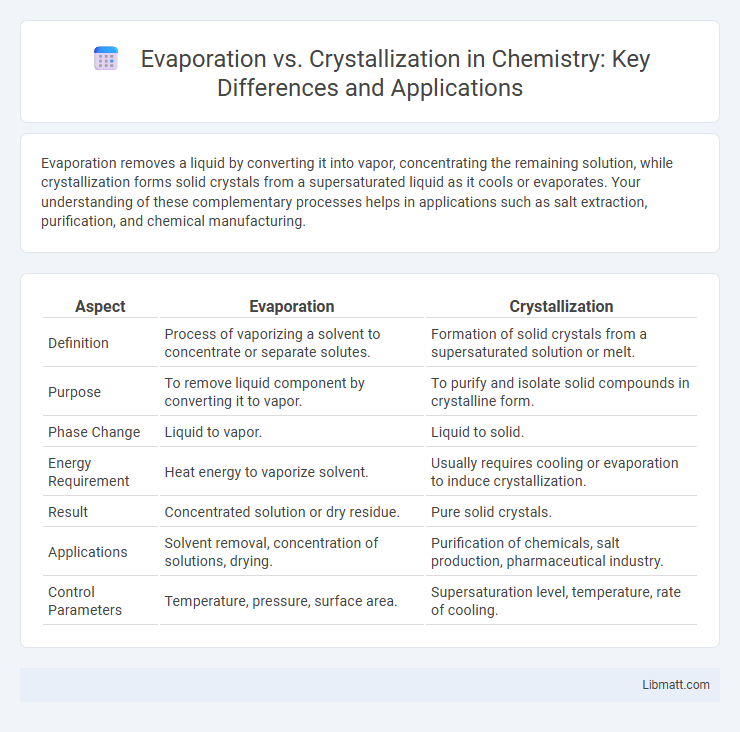
| Aspect | Evaporation | Crystallization |
|---|---|---|
| Definition | Process of vaporizing a solvent to concentrate or separate solutes. | Formation of solid crystals from a supersaturated solution or melt. |
| Purpose | To remove liquid component by converting it to vapor. | To purify and isolate solid compounds in crystalline form. |
| Phase Change | Liquid to vapor. | Liquid to solid. |
| Energy Requirement | Heat energy to vaporize solvent. | Usually requires cooling or evaporation to induce crystallization. |
| Result | Concentrated solution or dry residue. | Pure solid crystals. |
| Applications | Solvent removal, concentration of solutions, drying. | Purification of chemicals, salt production, pharmaceutical industry. |
| Control Parameters | Temperature, pressure, surface area. | Supersaturation level, temperature, rate of cooling. |
Introduction to Evaporation and Crystallization
Evaporation is the process where liquid molecules transition to a gas phase, typically driven by heat, causing the liquid to gradually reduce in volume. Crystallization occurs when dissolved substances in a liquid form solid crystals as the solvent evaporates or cools, concentrating the solute. Understanding these processes helps you optimize separation techniques in chemical and industrial applications.
Defining Evaporation: Processes and Principles
Evaporation is a physical process where a liquid transitions into a vapor at temperatures below its boiling point, driven by surface molecule energy overcoming intermolecular forces. This process is influenced by factors such as temperature, surface area, humidity, and airflow, which affect the rate of vaporization. Understanding the principles of evaporation is essential for applications in chemical engineering, environmental science, and industrial drying techniques.
Understanding Crystallization: Key Concepts
Crystallization is the process where dissolved substances come out of solution to form solid crystals, typically occurring when a solution becomes supersaturated. Unlike evaporation, which removes solvent to concentrate the solution, crystallization involves the orderly arrangement of molecules or ions into a structured lattice. Understanding the balance between temperature, concentration, and solubility is crucial for controlling your crystallization outcomes effectively.
Core Differences Between Evaporation and Crystallization
Evaporation involves the transformation of a liquid into vapor, primarily used to concentrate solutions by removing the solvent, while crystallization is the process where dissolved substances solidify into structured crystals as the solution becomes supersaturated. Your understanding of evaporation is essential for applications needing solvent removal without forming solid products, whereas crystallization is crucial for purifying compounds by controlling temperature and solute concentration. Both processes differ fundamentally in their outcomes: evaporation produces vapor, and crystallization yields solid crystals.
Scientific Mechanisms Behind Evaporation
Evaporation involves the transformation of liquid molecules into vapor as they gain sufficient kinetic energy to overcome intermolecular forces at the liquid's surface. This process occurs at temperatures below the liquid's boiling point and is influenced by factors such as temperature, surface area, and humidity. Understanding the scientific mechanisms of evaporation helps you optimize processes like drying, cooling, and solution concentration in various industrial and environmental contexts.
Scientific Mechanisms Behind Crystallization
Crystallization occurs when a solution becomes supersaturated, causing solute molecules to arrange into a highly ordered solid structure due to decreased solubility or temperature changes. Unlike evaporation, which simply removes solvent to concentrate the solution, crystallization involves nucleation and crystal growth processes driven by thermodynamic stability. Understanding these scientific mechanisms helps you control and optimize crystal purity and size in various industrial and laboratory applications.
Industrial Applications of Evaporation
Evaporation plays a critical role in industrial applications such as the concentration of solutions in chemical manufacturing, food processing, and wastewater treatment. It enables the separation of solvents from solutes, allowing for the recovery of valuable materials and reduction of waste volume. Industries commonly use multi-effect evaporators and vacuum evaporators to enhance energy efficiency and optimize production processes.
Industrial Applications of Crystallization
Crystallization is widely used in industries such as pharmaceuticals, chemicals, and food processing to purify substances and create solid crystals from liquid solutions, enabling precise control over product quality and composition. Unlike evaporation, which primarily concentrates solutions by removing solvents, crystallization facilitates separation based on solubility differences, making it essential for producing high-purity compounds like active pharmaceutical ingredients (APIs) and sugar crystals. Your industrial processes benefit from crystallization's ability to enhance material properties, optimize yield, and ensure consistency in large-scale manufacturing.
Factors Influencing Evaporation and Crystallization
Temperature, humidity, and surface area significantly influence evaporation rates by affecting the energy available for water molecules to transition into vapor. Crystallization depends primarily on supersaturation levels, cooling rate, and the presence of nucleation sites, which dictate crystal size and purity. Both processes are also impacted by pressure and the chemical nature of the solvent or solution involved.
Choosing Between Evaporation and Crystallization: Practical Considerations
Choosing between evaporation and crystallization depends on the desired purity and form of the final product; evaporation is ideal for concentrating solutions by removing solvents, while crystallization is preferred for obtaining pure solid crystals from a solution. Factors such as the solubility of the compound, temperature sensitivity, and the scale of the process also influence the choice. Practical considerations include process efficiency, energy consumption, and ease of separation in industrial or laboratory settings.
Evaporation vs crystallization Infographic
 libmatt.com
libmatt.com