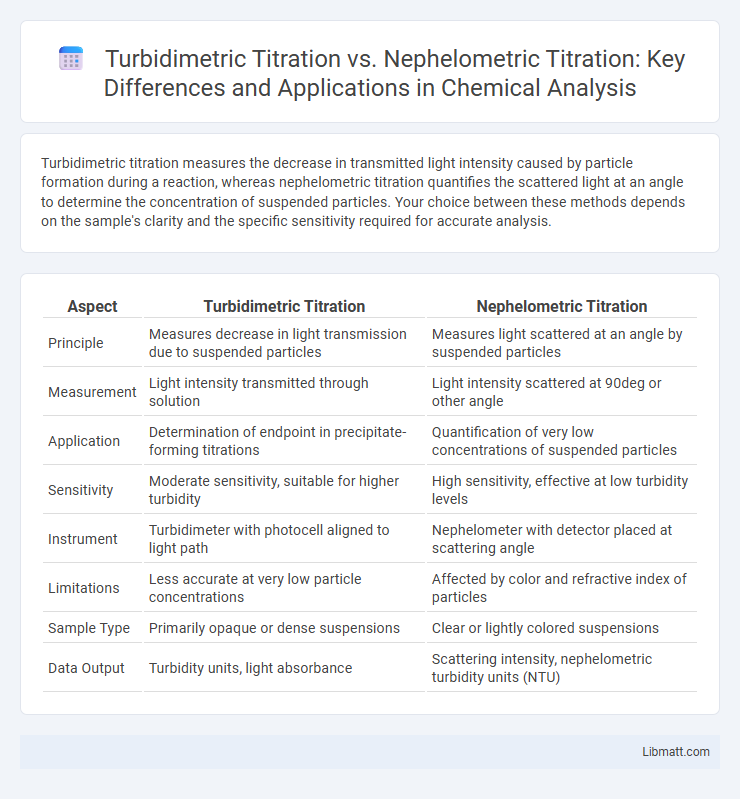
Turbidimetric titration measures the decrease in transmitted light intensity caused by particle formation during a reaction, whereas nephelometric titration quantifies the scattered light at an angle to determine the concentration of suspended particles. Your choice between these methods depends on the sample's clarity and the specific sensitivity required for accurate analysis.
Table of Comparison
| Aspect | Turbidimetric Titration | Nephelometric Titration |
|---|---|---|
| Principle | Measures decrease in light transmission due to suspended particles | Measures light scattered at an angle by suspended particles |
| Measurement | Light intensity transmitted through solution | Light intensity scattered at 90deg or other angle |
| Application | Determination of endpoint in precipitate-forming titrations | Quantification of very low concentrations of suspended particles |
| Sensitivity | Moderate sensitivity, suitable for higher turbidity | High sensitivity, effective at low turbidity levels |
| Instrument | Turbidimeter with photocell aligned to light path | Nephelometer with detector placed at scattering angle |
| Limitations | Less accurate at very low particle concentrations | Affected by color and refractive index of particles |
| Sample Type | Primarily opaque or dense suspensions | Clear or lightly colored suspensions |
| Data Output | Turbidity units, light absorbance | Scattering intensity, nephelometric turbidity units (NTU) |
Introduction to Turbidimetric and Nephelometric Titration
Turbidimetric titration measures the decrease in solution turbidity as a precipitate forms, while nephelometric titration quantifies the light scattered by suspended particles in the sample. Both techniques rely on detecting changes in particle concentration but differ in their optical detection methods--turbidimetry uses transmitted light intensity, whereas nephelometry measures scattered light at an angle. Understanding these distinctions helps optimize analytical accuracy and sensitivity in your titration experiments.
Principle of Turbidimetric Titration
Turbidimetric titration measures the decrease in turbidity or cloudiness of a solution caused by the formation of a precipitate during the titration process, using a photometer to detect changes in light transmission. The principle is based on the inverse relationship between turbidity and transmitted light intensity, where the endpoint is identified by a significant change in turbidity indicating complete reaction. This technique is effective for quantifying analytes that form insoluble precipitates, such as sulfate or chloride ions.
Principle of Nephelometric Titration
Nephelometric titration measures the intensity of scattered light caused by the formation of fine precipitates during a chemical reaction, directly correlating turbidity to analyte concentration. This technique relies on detecting light scattered at specific angles, allowing precise endpoint determination even in low concentrations with minimal interference. Your choice between turbidimetric and nephelometric titration depends on the sensitivity required and the physical properties of the precipitate formed.
Equipment and Instrumentation Used
Turbidimetric titration utilizes a turbidimeter equipped with a photodetector and a light source, typically a tungsten or LED lamp, to measure the decrease in light transmission as particle concentration increases. Nephelometric titration employs a nephelometer designed to detect scattered light at an angle, often 90 degrees, using photomultiplier tubes or photodiodes to quantify suspended particles in the solution. Both techniques require precise calibration standards and stable optical components to ensure accurate measurement of turbidity or nephelometry during titration analysis.
Sample Preparation and Handling
Sample preparation for turbidimetric titration requires careful dilution and homogenization to ensure consistent particle suspension, preventing sedimentation that can affect turbidity measurements. In nephelometric titration, samples must be handled to minimize light scattering interference by removing large particulates or using appropriate filters, maintaining stable colloidal dispersions. Both techniques demand stringent control of sample temperature and pH to preserve the physicochemical properties influencing light scattering and turbidity detection.
Analytical Procedures: Step-by-Step Comparison
In turbidimetric titration, the analytical procedure involves adding a titrant gradually to a colloidal solution while continuously measuring the decrease in turbidity using a turbidimeter until a clear endpoint is reached. Nephelometric titration measures the increase in scattered light intensity at a specific angle as the titrant forms an insoluble precipitate, with a nephelometer detecting changes to determine the equivalence point. Both methods require careful calibration of instruments, precise sample preparation, and controlled titrant addition to ensure accurate quantification of analytes based on changes in light transmission or scattering.
Applications in Chemical and Biological Analysis
Turbidimetric titration measures the decrease in turbidity caused by precipitation reactions, making it ideal for determining concentrations of ions like sulfate and chloride in water samples. Nephelometric titration quantifies scattered light from suspended particles, providing high sensitivity for trace protein, antigen, and antibody detection in clinical immunoassays. Both methods are essential in chemical and biological analysis for monitoring reaction endpoints and quantifying analytes in environmental, pharmaceutical, and clinical laboratories.
Advantages and Limitations of Each Technique
Turbidimetric titration offers high sensitivity in detecting endpoint changes by measuring the decrease in solution turbidity, making it suitable for reactions producing fine precipitates; however, it can be limited by interference from colored or opaque samples. Nephelometric titration provides advantages in detecting light scattered by suspended particles, enabling precise quantification of small amounts of analyte in colloidal suspensions, yet it may struggle with reproducibility in highly turbid or fluctuating samples. Your choice between these techniques depends on the sample matrix, required sensitivity, and the nature of particulates involved in the titration.
Sensitivity, Accuracy, and Reproducibility
Turbidimetric titration offers higher sensitivity by measuring the decrease in light transmission as particles form, suitable for detecting subtle changes in particle concentration. Nephelometric titration provides enhanced accuracy through direct measurement of scattered light at a specific angle, reducing interference from background turbulence. Your choice between these methods depends on the critical need for reproducibility, with nephelometry typically yielding more consistent results across repeated trials.
Choosing Between Turbidimetric and Nephelometric Titration
Choosing between turbidimetric and nephelometric titration depends on the particle size and concentration in your sample, as turbidimetric titration measures the decrease in light transmission while nephelometric titration detects scattered light at specific angles. Turbidimetry is ideal for samples with higher turbidity and larger particles, offering simplicity and quick readings, whereas nephelometry provides greater sensitivity for low concentrations and smaller particles by measuring light scatter intensity. Your choice should consider the analyte characteristics and required detection limits to ensure accurate and reliable titration results.
Turbidimetric titration vs nephelometric titration Infographic
 libmatt.com
libmatt.com