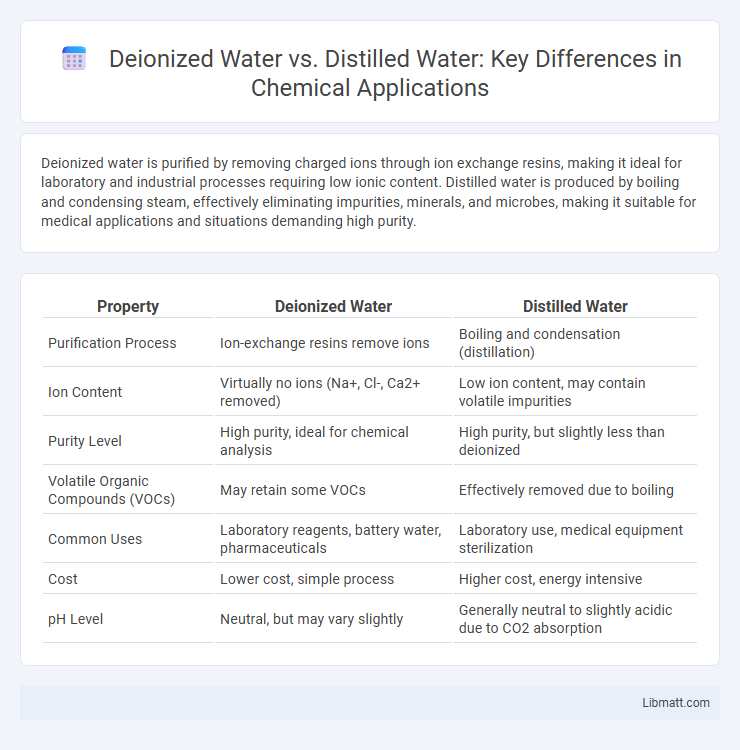
Deionized water is purified by removing charged ions through ion exchange resins, making it ideal for laboratory and industrial processes requiring low ionic content. Distilled water is produced by boiling and condensing steam, effectively eliminating impurities, minerals, and microbes, making it suitable for medical applications and situations demanding high purity.
Table of Comparison
| Property | Deionized Water | Distilled Water |
|---|---|---|
| Purification Process | Ion-exchange resins remove ions | Boiling and condensation (distillation) |
| Ion Content | Virtually no ions (Na+, Cl-, Ca2+ removed) | Low ion content, may contain volatile impurities |
| Purity Level | High purity, ideal for chemical analysis | High purity, but slightly less than deionized |
| Volatile Organic Compounds (VOCs) | May retain some VOCs | Effectively removed due to boiling |
| Common Uses | Laboratory reagents, battery water, pharmaceuticals | Laboratory use, medical equipment sterilization |
| Cost | Lower cost, simple process | Higher cost, energy intensive |
| pH Level | Neutral, but may vary slightly | Generally neutral to slightly acidic due to CO2 absorption |
Introduction to Deionized and Distilled Water
Deionized water is produced by removing ions through ion exchange resins, resulting in water with very low electrical conductivity and minimal dissolved minerals. Distilled water is created by boiling water and condensing the steam, eliminating impurities, bacteria, and most minerals. Both types are used in laboratories and industrial applications where purity is essential, but your choice depends on the specific contaminants you need to eliminate.
What is Deionized Water?
Deionized water is purified by removing mineral ions such as sodium, calcium, iron, and copper through an ion exchange process, resulting in water with very low conductivity. This type of water is commonly used in laboratory settings, pharmaceuticals, and industrial applications where the presence of ions can interfere with processes or products. Unlike distilled water, which is purified by boiling and condensation, deionized water specifically targets and eliminates ionic impurities without removing non-ionic contaminants.
What is Distilled Water?
Distilled water is purified through the process of boiling and subsequent condensation, which removes impurities, minerals, and contaminants. This method ensures the water is free from dissolved solids, making it ideal for laboratory experiments, medical uses, and devices requiring mineral-free water. Your applications requiring high purity benefit from distilled water's effective removal of organic and inorganic substances.
Deionization vs Distillation: Key Processes
Deionization removes dissolved ionic impurities by exchanging positive and negative ions using resin beds, producing water with low mineral content but not removing non-ionic contaminants. Distillation involves boiling water to create steam and then condensing it back into liquid, effectively eliminating a broad range of contaminants including bacteria, viruses, and non-ionic substances. Each process serves different purity requirements: deionization is faster and cost-effective for industrial use, while distillation provides higher purity suitable for laboratory and medical applications.
Purity Levels: Deionized vs Distilled Water
Deionized water achieves high purity by removing mineral ions like sodium, calcium, iron, and copper through ion-exchange resins, resulting in a resistivity typically around 18.2 MO*cm. Distilled water attains purity by boiling and condensing steam, effectively eliminating most impurities, including bacteria and organic compounds, but trace volatile contaminants may remain. While both types reach high purity, deionized water offers more consistent ion removal, whereas distilled water provides broader removal of non-ionic substances.
Common Uses of Deionized Water
Deionized water is commonly used in laboratories, pharmaceutical manufacturing, and electronics cleaning because it effectively removes mineral ions that can interfere with sensitive processes and equipment. Its high purity makes it ideal for preparing solutions, rinsing lab glassware, and cooling systems where mineral deposits could cause damage. You can rely on deionized water to ensure contamination-free results in industrial and scientific applications.
Common Uses of Distilled Water
Distilled water is commonly used in medical settings, laboratory experiments, and automotive cooling systems due to its high purity and lack of contaminants. Its use in steam irons and humidifiers prevents mineral build-up, extending the lifespan of these appliances. You can also rely on distilled water for cosmetic formulations where purity is crucial to avoid skin irritation.
Benefits and Drawbacks of Deionized Water
Deionized water offers high purity by removing mineral ions, making it ideal for laboratory applications, electronics manufacturing, and automotive cooling systems where mineral deposits can cause damage. However, its lack of ions also means it is more corrosive to metals and lacks essential minerals necessary for biological processes, limiting its use in drinking water or certain industrial operations. The cost of producing deionized water can be higher compared to other purification methods, restricting its widespread utilization.
Benefits and Drawbacks of Distilled Water
Distilled water offers high purity by removing impurities and minerals through boiling and condensation, making it ideal for medical and laboratory use. However, the lack of minerals in distilled water can lead to a flat taste and may leach minerals from your body if consumed regularly. While it ensures cleanliness, it may not provide the health benefits associated with mineral-rich water types.
Which is Better: Deionized or Distilled Water?
Deionized water contains ions removed through ion exchange resins, resulting in very low mineral content ideal for laboratory and industrial applications. Distilled water undergoes boiling and condensation, effectively removing impurities and microorganisms, making it suitable for medical and drinking purposes. The choice between deionized and distilled water depends on the specific use case, with deionized water preferred for electronics and pharmaceuticals and distilled water favored for consumption and sterilization.
Deionized water vs distilled water Infographic
 libmatt.com
libmatt.com