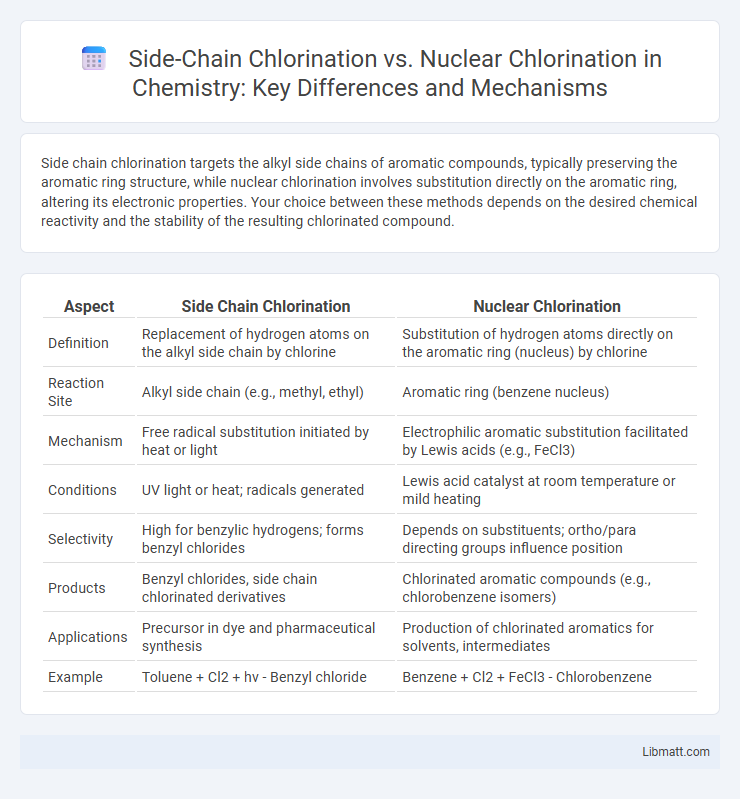
Side chain chlorination targets the alkyl side chains of aromatic compounds, typically preserving the aromatic ring structure, while nuclear chlorination involves substitution directly on the aromatic ring, altering its electronic properties. Your choice between these methods depends on the desired chemical reactivity and the stability of the resulting chlorinated compound.
Table of Comparison
| Aspect | Side Chain Chlorination | Nuclear Chlorination |
|---|---|---|
| Definition | Replacement of hydrogen atoms on the alkyl side chain by chlorine | Substitution of hydrogen atoms directly on the aromatic ring (nucleus) by chlorine |
| Reaction Site | Alkyl side chain (e.g., methyl, ethyl) | Aromatic ring (benzene nucleus) |
| Mechanism | Free radical substitution initiated by heat or light | Electrophilic aromatic substitution facilitated by Lewis acids (e.g., FeCl3) |
| Conditions | UV light or heat; radicals generated | Lewis acid catalyst at room temperature or mild heating |
| Selectivity | High for benzylic hydrogens; forms benzyl chlorides | Depends on substituents; ortho/para directing groups influence position |
| Products | Benzyl chlorides, side chain chlorinated derivatives | Chlorinated aromatic compounds (e.g., chlorobenzene isomers) |
| Applications | Precursor in dye and pharmaceutical synthesis | Production of chlorinated aromatics for solvents, intermediates |
| Example | Toluene + Cl2 + hv - Benzyl chloride | Benzene + Cl2 + FeCl3 - Chlorobenzene |
Introduction to Chlorination in Organic Compounds
Chlorination in organic compounds involves the substitution of hydrogen atoms with chlorine atoms, crucially affecting molecular reactivity and properties. Side chain chlorination specifically targets the alkyl side chains of aromatic compounds, typically via free radical mechanisms, producing benzylic chlorides important in synthetic chemistry. Nuclear chlorination, in contrast, substitutes chlorine directly onto the aromatic ring through electrophilic aromatic substitution, influencing substitution patterns and electronic effects in aromatic systems.
What is Side Chain Chlorination?
Side chain chlorination involves the substitution of hydrogen atoms on the alkyl side chain of an aromatic compound with chlorine atoms, typically initiated by free radicals under UV light or heat. This process selectively targets the side chain rather than the aromatic nucleus, preserving the ring structure while introducing chlorine atoms to increase reactivity or alter physical properties. Understanding side chain chlorination is crucial for your applications in pharmaceuticals, agrochemicals, and polymer industries where modified side chains play a significant role in product performance.
What is Nuclear Chlorination?
Nuclear chlorination is a chemical reaction where chlorine atoms substitute hydrogen atoms directly on the aromatic ring (nucleus) of an organic compound, typically an aromatic hydrocarbon like benzene. This electrophilic aromatic substitution specifically targets the aromatic core, altering the compound's electronic properties and often affecting its reactivity and stability. Nuclear chlorination contrasts with side chain chlorination, which involves substitution on alkyl side chains rather than the aromatic nucleus.
Mechanistic Differences: Side Chain vs Nuclear Chlorination
Side chain chlorination involves the substitution of hydrogen atoms on the alkyl side chains of aromatic compounds through a radical chain mechanism initiated by light or heat, producing benzylic chlorides. Nuclear chlorination occurs directly on the aromatic ring via electrophilic aromatic substitution, where the chlorine acts as an electrophile and the aromatic p system stabilizes the intermediate arenium ion. Understanding these mechanistic differences helps you predict product distribution and reactivity in chlorination reactions.
Key Reactants and Conditions for Chlorination Types
Side chain chlorination involves the substitution of hydrogen atoms on the alkyl side chain of aromatic compounds, typically requiring free radical initiators such as UV light or heat and chlorine gas as key reactants. Nuclear chlorination targets hydrogen atoms directly attached to the aromatic ring (nucleus), often catalyzed by Lewis acids like FeCl3 or AlCl3 under milder temperature conditions. Your choice of chlorination type depends on the desired product and reaction conditions, with side chain chlorination favoring radical pathways and nuclear chlorination proceeding via electrophilic aromatic substitution.
Product Outcomes: Comparative Analysis
Side chain chlorination primarily yields benzylic chlorides, which serve as valuable intermediates in organic synthesis, while nuclear chlorination results in chlorinated aromatic compounds with substitution occurring on the ring itself. Side chain chlorination typically produces products with higher regioselectivity and fewer positional isomers, enhancing purification and application efficiency. When considering Your synthesis goals, choosing between these methods depends on whether the target compound requires benzylic functionalization or direct aromatic substitution.
Selectivity and Regioselectivity Insights
Side chain chlorination exhibits higher selectivity by targeting benzylic or allylic hydrogens, leading to specific substitution at the side chain rather than the aromatic ring. Nuclear chlorination, influenced by electron density and resonance effects, shows regioselectivity favoring ortho and para positions relative to activating groups on the aromatic system. Understanding these regioselectivity insights allows you to predict and control chlorination outcomes in complex organic syntheses.
Industrial Applications of Each Chlorination Method
Side chain chlorination is widely used in the production of high-performance polymers and specialty chemicals, such as chloromethylated polystyrene resins utilized in ion exchange and catalysis. Nuclear chlorination is essential in the manufacture of aromatic chlorides like chlorobenzene, a precursor for herbicides, dyes, and pharmaceuticals in large-scale chemical industries. Industrial applications favor side chain chlorination for modifying polymer properties while nuclear chlorination is preferred for synthesizing chlorinated aromatic intermediates crucial to agrochemical and pharmaceutical sectors.
Environmental and Safety Considerations
Side chain chlorination generally produces fewer persistent organic pollutants, making it a more environmentally favorable option compared to nuclear chlorination, which can generate hazardous poly-chlorinated aromatic compounds. You should consider that nuclear chlorination involves direct substitution on the aromatic ring, often leading to more toxic and less biodegradable byproducts with greater ecological impact. Ensuring proper handling and containment measures is crucial for both processes, but the higher risk associated with nuclear chlorination demands stricter safety protocols to prevent environmental contamination and worker exposure.
Conclusion: Choosing the Right Chlorination Process
Side chain chlorination selectively replaces hydrogen atoms on alkyl side chains of aromatic compounds, preserving the aromatic ring's integrity, making it ideal for producing intermediates in pharmaceuticals and agrochemicals. Nuclear chlorination targets the aromatic ring directly, leading to substitution on the ring and altering its electronic properties, often used in synthesizing chlorinated aromatic compounds like chlorobenzene. Selecting the appropriate chlorination process depends on the desired chemical structure and application, with side chain chlorination preferred for maintaining ring stability and nuclear chlorination chosen for modifying ring reactivity.
side chain chlorination vs nuclear chlorination Infographic
 libmatt.com
libmatt.com