Electrodialysis uses ion-exchange membranes and an electric field to separate charged particles from water, making it highly efficient for desalination and industrial wastewater treatment. Capacitive deionization removes ions by adsorbing them onto charged porous electrodes, offering a lower-energy alternative ideal for brackish water and small-scale water purification systems.
Table of Comparison
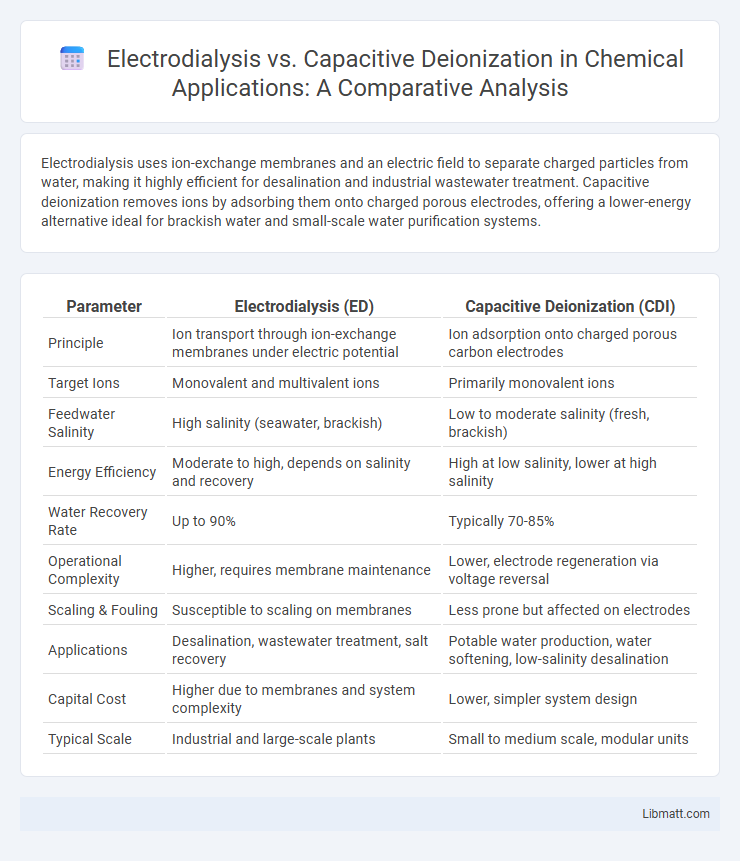
| Parameter | Electrodialysis (ED) | Capacitive Deionization (CDI) |
|---|---|---|
| Principle | Ion transport through ion-exchange membranes under electric potential | Ion adsorption onto charged porous carbon electrodes |
| Target Ions | Monovalent and multivalent ions | Primarily monovalent ions |
| Feedwater Salinity | High salinity (seawater, brackish) | Low to moderate salinity (fresh, brackish) |
| Energy Efficiency | Moderate to high, depends on salinity and recovery | High at low salinity, lower at high salinity |
| Water Recovery Rate | Up to 90% | Typically 70-85% |
| Operational Complexity | Higher, requires membrane maintenance | Lower, electrode regeneration via voltage reversal |
| Scaling & Fouling | Susceptible to scaling on membranes | Less prone but affected on electrodes |
| Applications | Desalination, wastewater treatment, salt recovery | Potable water production, water softening, low-salinity desalination |
| Capital Cost | Higher due to membranes and system complexity | Lower, simpler system design |
| Typical Scale | Industrial and large-scale plants | Small to medium scale, modular units |
Introduction to Electrodialysis and Capacitive Deionization
Electrodialysis utilizes ion-exchange membranes and an electric potential to separate ions from water, making it effective for desalination and wastewater treatment. Capacitive deionization, on the other hand, employs porous electrodes to adsorb and remove charged ions, offering energy-efficient ion removal in low-salinity water. Your choice between Electrodialysis and Capacitive Deionization depends on water quality and treatment goals, with both technologies advancing sustainable water purification.
Working Principles: Electrodialysis Overview
Electrodialysis operates by utilizing ion-exchange membranes and an electric potential to selectively separate ions from water, effectively desalinating or demineralizing the solution. This process involves the migration of cations and anions through respective membranes towards electrodes of opposite charge, resulting in purified water streams and concentrated brine. Understanding electrodialysis can help you optimize water treatment systems, especially where efficient ion separation and recovery are critical.
Capacitive Deionization: Mechanism Explained
Capacitive deionization (CDI) operates by applying an electrical potential difference across porous carbon electrodes to adsorb and remove ions from water, effectively reducing salinity. This electrochemical mechanism involves the formation of electrical double layers where charged ions are attracted and held on electrode surfaces without involving chemical reactions. Your water purification process benefits from CDI's energy efficiency and ability to selectively remove ions, especially in low to moderate salinity conditions.
Key Differences Between Electrodialysis and CDI
Electrodialysis uses ion-selective membranes and an electric field to separate charged ions from water, making it highly effective for desalination and brackish water treatment. Capacitive deionization (CDI) employs porous carbon electrodes to adsorb ions electrostatically, which is more energy-efficient for low-salinity water but less effective with high-salinity feed. Your choice depends on factors like feed water salinity, energy consumption, and system complexity, with electrodialysis favoring higher ionic strengths and CDI preferred for softer water applications.
Ion Removal Efficiency: Comparative Analysis
Electrodialysis achieves higher ion removal efficiency by using ion-selective membranes that selectively transport ions under an electric field, typically removing up to 99% of dissolved salts. Capacitive deionization relies on electro-sorption in porous carbon electrodes, removing ions with efficiencies around 70-90%, making it more effective for low to moderate salinity water. Your choice depends on the specific water composition and desired purification level, with electrodialysis excelling in high salinity situations and capacitive deionization being advantageous for energy-efficient, less concentrated ion removal.
Energy Consumption in Electrodialysis vs CDI
Electrodialysis typically exhibits higher energy consumption due to the continuous electrical potential required to drive ion migration through selective membranes. Capacitive deionization (CDI) offers lower energy usage by employing electrostatic adsorption of ions onto porous electrodes, enabling energy recovery during regeneration phases. Energy efficiency in CDI can reach up to 70-80%, whereas electrodialysis efficiency commonly ranges between 40-60%, making CDI more suitable for low to moderate salinity water treatment.
Operational and Maintenance Considerations
Electrodialysis systems require regular membrane cleaning and replacement to prevent fouling and scaling, which can increase downtime and operational costs. Capacitive deionization features simpler maintenance with fewer consumable parts, as electrodes primarily need periodic regeneration or replacement. Your choice depends on balancing these operational demands against water quality requirements and system longevity.
Applications in Water Treatment and Desalination
Electrodialysis is widely utilized in industrial-scale desalination and brackish water treatment due to its efficiency in selectively removing ions using ion-exchange membranes. Capacitive deionization offers a promising solution for low-salinity water purification and wastewater reuse by employing electrically charged electrodes to adsorb ions. Your choice between these technologies depends on water salinity levels, desired purity, and operational costs in specific water treatment applications.
Cost Analysis and Economic Feasibility
Electrodialysis typically involves higher initial capital costs due to expensive ion-exchange membranes and complex system components, but it offers lower operational expenses and longer membrane lifespans, making it economical for high-salinity water treatment. Capacitive deionization (CDI) has lower upfront investment and simpler design, ideal for low-salinity applications, but its energy consumption and electrode replacement frequency can increase long-term expenses. Your choice depends on water quality and treatment scale, balancing capital expenditure against operational costs for optimal economic feasibility.
Future Prospects and Technological Advancements
Electrodialysis technology continues to evolve with advances in membrane materials and energy efficiency, promising enhanced ion selectivity and reduced operational costs for large-scale desalination. Capacitive deionization is gaining momentum due to innovations in electrode design and scalable modular systems, making it increasingly viable for portable and small-scale water treatment applications. Your choice between these methods will depend on future improvements aligning with specific water quality requirements and energy consumption goals.
Electrodialysis vs capacitive deionization Infographic
 libmatt.com
libmatt.com