Adsorption involves the adhesion of molecules onto a surface, while absorption refers to the process where one substance is fully taken up into the bulk of another. Understanding the difference between adsorption and absorption helps you choose the right method for applications like filtration, purification, and chemical processing.
Table of Comparison
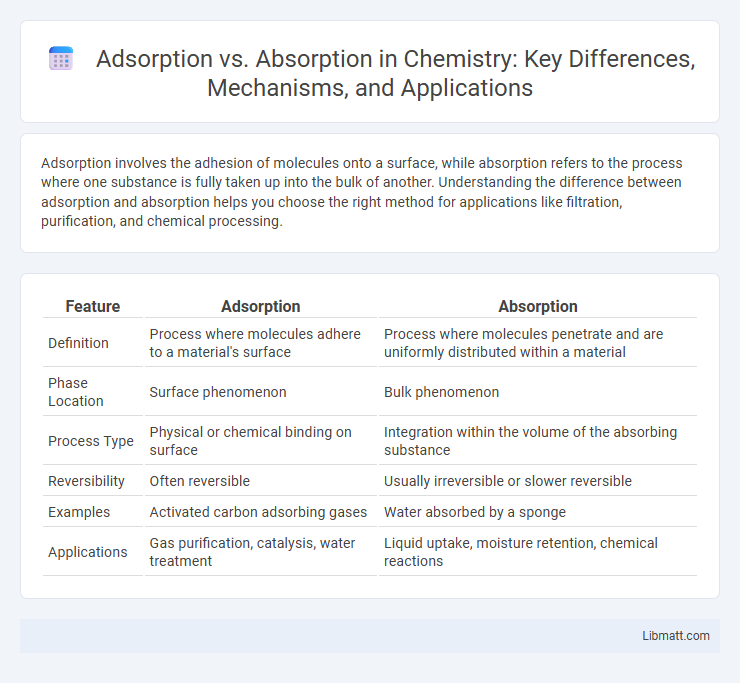
| Feature | Adsorption | Absorption |
|---|---|---|
| Definition | Process where molecules adhere to a material's surface | Process where molecules penetrate and are uniformly distributed within a material |
| Phase Location | Surface phenomenon | Bulk phenomenon |
| Process Type | Physical or chemical binding on surface | Integration within the volume of the absorbing substance |
| Reversibility | Often reversible | Usually irreversible or slower reversible |
| Examples | Activated carbon adsorbing gases | Water absorbed by a sponge |
| Applications | Gas purification, catalysis, water treatment | Liquid uptake, moisture retention, chemical reactions |
Introduction to Adsorption and Absorption
Adsorption is a surface phenomenon where molecules accumulate on the surface of a solid or liquid, while absorption involves the entire volume of a material integrating the molecules. Adsorption is typically reversible and depends on surface properties and interactions, whereas absorption results in a uniform distribution within the absorbing medium. Understanding these differences is crucial in applications like catalysis, purification, and material science.
Fundamental Definitions
Adsorption is the process where molecules adhere only to the surface of a solid or liquid, forming a molecular or atomic film. Absorption involves the penetration of a substance into the bulk phase of a solid or liquid, where it is uniformly distributed throughout the material. The key difference lies in adsorption being a surface phenomenon while absorption is a volume phenomenon.
Key Differences Between Adsorption and Absorption
Adsorption is a surface phenomenon where molecules accumulate on the surface of a solid or liquid, while absorption involves the entire volume of the material, with substances penetrating into the bulk phase. Adsorption is typically reversible and depends on surface area and interaction forces, whereas absorption results in a homogeneous mixture and often permanent incorporation of the absorbed substance. These fundamental differences impact applications in catalysis, filtration, and chemical engineering processes.
Mechanisms of Adsorption
Adsorption involves the accumulation of molecules from a gas or liquid phase onto the surface of a solid or liquid, driven primarily by physical forces such as van der Waals interactions or chemical bonding processes like chemisorption. This surface phenomenon contrasts with absorption, where molecules penetrate the entire volume of the absorbing material. Understanding the mechanisms of adsorption, including physisorption with weak intermolecular forces and chemisorption involving stronger, often irreversible bonds, is crucial for applications in catalysis, filtration, and surface coating technologies.
Mechanisms of Absorption
Absorption involves the process where molecules or atoms penetrate into the bulk phase of a solid or liquid material, typically driven by diffusion and solubility differences. In chemical absorption, the absorbed substance reacts chemically with the absorbent, forming new compounds, while physical absorption relies on intermolecular forces without chemical change. The efficiency of absorption depends on factors such as temperature, pressure, surface area, and the nature of the absorbent and absorbate.
Factors Affecting Adsorption
Surface area, temperature, and pressure critically influence adsorption efficiency, with higher surface areas providing more active sites for adsorbate molecules. Adsorbent porosity and chemical nature dictate the extent of adsorption through physical or chemical interactions, while temperature variations can either enhance or reduce adsorption depending on whether the process is exothermic or endothermic. Pressure directly affects the adsorption equilibrium, following Henry's law for gases where increased pressure generally leads to increased adsorption capacity.
Factors Influencing Absorption
Absorption depends heavily on factors such as temperature, pressure, concentration gradient, and the nature of the absorbent and absorbate materials. Higher temperatures typically increase the kinetic energy of molecules, enhancing the absorption rate for gases and liquids into solids or liquids. The surface area and porosity of the absorbent also play critical roles in determining the efficiency and capacity of absorption processes.
Real-World Applications of Adsorption
Adsorption plays a crucial role in water purification by removing contaminants such as heavy metals and organic pollutants using activated carbon filters. Industrial gas separation processes widely utilize adsorption to selectively capture gases like carbon dioxide and nitrogen, enhancing efficiency and reducing emissions. In pharmaceutical manufacturing, adsorption aids in drug formulation and purification, ensuring product quality by selectively binding specific molecules.
Common Uses of Absorption
Absorption is widely used in industries such as chemical manufacturing, where gases or liquids are taken up by solids or liquids for purification or separation purposes. In environmental engineering, absorption helps in removing pollutants from air or water, such as in activated carbon filters that absorb contaminants. Medical applications include drug delivery systems, where absorption facilitates the uptake of medications into the bloodstream for therapeutic effects.
Summary: Choosing Between Adsorption and Absorption
Adsorption involves the adhesion of molecules onto a surface, while absorption is the process where a substance penetrates and is uniformly distributed within another material. Your choice between adsorption and absorption depends on factors like the material's surface properties, the nature of the substances involved, and the intended application, such as filtration or chemical reactions. Adsorption is typically favored for surface-level interactions, whereas absorption suits scenarios requiring integration into the bulk material.
adsorption vs absorption Infographic
 libmatt.com
libmatt.com