Pyrometallurgy uses high temperatures to extract and refine metals through processes like smelting and roasting, while hydrometallurgy relies on aqueous chemistry, using solvents and leaching agents to dissolve metals from ores. Your choice between these methods depends on ore type, environmental considerations, and energy efficiency.
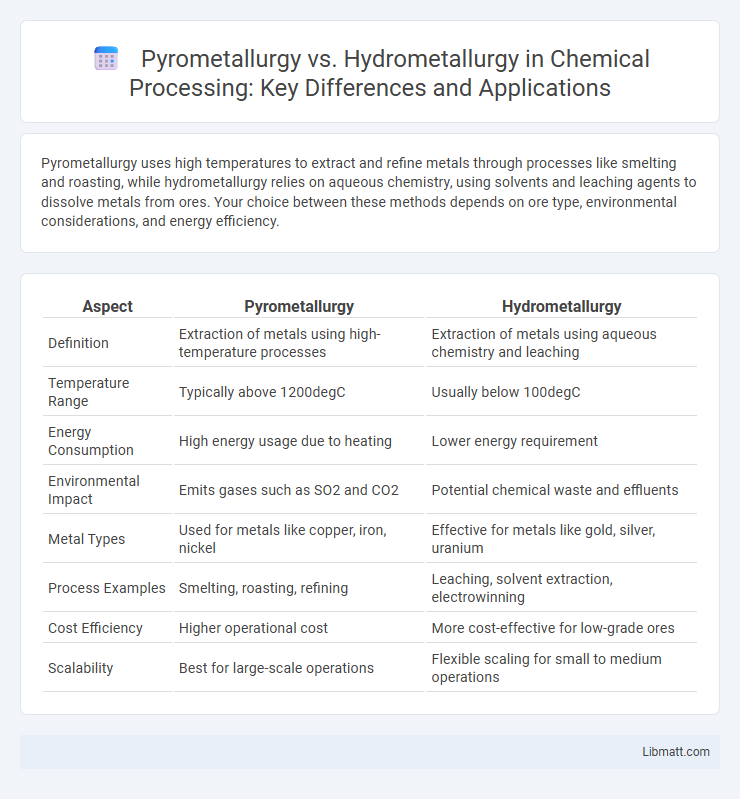
Table of Comparison
| Aspect | Pyrometallurgy | Hydrometallurgy |
|---|---|---|
| Definition | Extraction of metals using high-temperature processes | Extraction of metals using aqueous chemistry and leaching |
| Temperature Range | Typically above 1200degC | Usually below 100degC |
| Energy Consumption | High energy usage due to heating | Lower energy requirement |
| Environmental Impact | Emits gases such as SO2 and CO2 | Potential chemical waste and effluents |
| Metal Types | Used for metals like copper, iron, nickel | Effective for metals like gold, silver, uranium |
| Process Examples | Smelting, roasting, refining | Leaching, solvent extraction, electrowinning |
| Cost Efficiency | Higher operational cost | More cost-effective for low-grade ores |
| Scalability | Best for large-scale operations | Flexible scaling for small to medium operations |
Introduction to Pyrometallurgy and Hydrometallurgy
Pyrometallurgy involves high-temperature processes to extract and refine metals by smelting and roasting ores, mainly used for metals like copper, iron, and nickel. Hydrometallurgy utilizes aqueous solutions and chemical reactions such as leaching, solvent extraction, and electrowinning to recover metals from ores or concentrates, commonly applied for precious metals like gold and silver. Both methods are integral to metallurgical engineering, offering tailored solutions based on ore characteristics and environmental considerations.
Fundamental Principles of Pyrometallurgy
Pyrometallurgy involves the thermal treatment of minerals and metallurgical ores to extract metals, relying on high-temperature processes such as smelting, roasting, and refining. It fundamentally depends on thermochemical reactions where heat induces chemical transformations, including reduction, oxidation, and slag formation, to separate metals from impurities. Core processes include blast furnace operation for iron extraction and reverberatory furnaces used in copper smelting, emphasizing temperature control and reaction kinetics for efficient metal recovery.
Key Mechanisms in Hydrometallurgical Processes
Hydrometallurgical processes rely on aqueous chemistry to extract metals through leaching, solvent extraction, and electrowinning, utilizing chemical reactions such as oxidation-reduction and complexation. Key mechanisms include selective dissolution of metals from ores or concentrates, followed by separation and purification steps that enhance metal recovery while minimizing environmental impact. Understanding these processes allows you to optimize metal extraction efficiently compared to pyrometallurgy, which depends on high-temperature thermal treatments.
Common Applications in Metal Extraction
Pyrometallurgy is widely used in extracting metals such as copper, nickel, and lead through high-temperature processes like smelting and roasting, making it essential for materials requiring thermal decomposition or reduction. Hydrometallurgy is preferred for metals like gold, silver, and zinc, utilizing aqueous solutions to leach and separate metals at lower temperatures, which is efficient for low-grade ores and environmentally sensitive operations. Both techniques are critical in the mining industry, with pyrometallurgy dominating bulk metal production and hydrometallurgy excelling in selective metal recovery and waste minimization.
Energy Consumption and Efficiency Comparison
Pyrometallurgy typically demands higher energy consumption due to high-temperature processes such as smelting and roasting, making it less energy-efficient compared to hydrometallurgy, which operates at lower temperatures using aqueous solutions. Hydrometallurgy offers improved energy efficiency by leveraging chemical leaching and solvent extraction, resulting in reduced greenhouse gas emissions and operational costs. Your choice between these methods should consider the trade-off between energy input and metal recovery efficiency based on the specific ore and environmental regulations.
Environmental Impact and Sustainability
Pyrometallurgy often involves high energy consumption and significant greenhouse gas emissions due to its reliance on high-temperature processes, which can impact air quality and contribute to climate change. Hydrometallurgy generally offers a more environmentally sustainable alternative by using aqueous solutions at lower temperatures, reducing energy use and emissions while enabling metal recovery with less waste. Your choice between these methods should consider the specific metal, processing scale, and sustainability goals to minimize environmental footprints effectively.
Advantages of Pyrometallurgy
Pyrometallurgy offers significant advantages including faster processing times and higher throughput compared to hydrometallurgy. It effectively treats complex and low-grade ores, enabling the recovery of metals like copper, nickel, and iron with high purity. Furthermore, pyrometallurgical processes often result in more environmentally stable slag and reduced risk of metal contamination.
Benefits of Hydrometallurgical Techniques
Hydrometallurgical techniques offer significant benefits including lower energy consumption compared to pyrometallurgy, making them more environmentally friendly and cost-effective. These methods enable selective metal recovery from low-grade ores and complex materials, enhancing resource efficiency and reducing waste. Furthermore, hydrometallurgy typically operates at lower temperatures, minimizing atmospheric emissions and improving overall process safety.
Challenges and Limitations of Each Method
Pyrometallurgy faces challenges such as high energy consumption, emission of pollutants, and difficulty in processing low-grade ores, which can impact environmental compliance and operational costs. Hydrometallurgy is limited by slower reaction rates, sensitivity to solution chemistry, and the need for large volumes of water, potentially causing waste disposal issues. Your selection between these methods should consider ore type, environmental regulations, and resource availability to mitigate their inherent limitations effectively.
Future Trends in Metallurgical Processes
Future trends in pyrometallurgy emphasize energy efficiency improvements and integration of renewable energy sources to reduce carbon emissions in high-temperature metal extraction. Hydrometallurgy advancements target enhanced solvent extraction techniques and bioleaching methods for more sustainable and selective recovery of critical metals from complex ores and industrial waste. The shift towards circular economy principles drives innovation in both fields to maximize resource recovery while minimizing environmental impact.
pyrometallurgy vs hydrometallurgy Infographic
 libmatt.com
libmatt.com