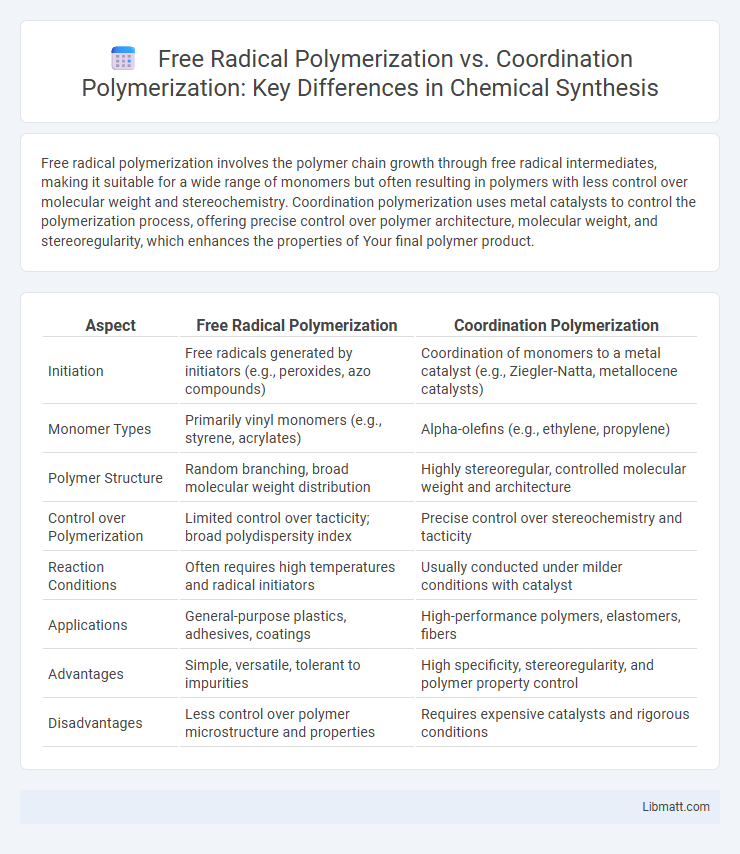
Free radical polymerization involves the polymer chain growth through free radical intermediates, making it suitable for a wide range of monomers but often resulting in polymers with less control over molecular weight and stereochemistry. Coordination polymerization uses metal catalysts to control the polymerization process, offering precise control over polymer architecture, molecular weight, and stereoregularity, which enhances the properties of Your final polymer product.
Table of Comparison
| Aspect | Free Radical Polymerization | Coordination Polymerization |
|---|---|---|
| Initiation | Free radicals generated by initiators (e.g., peroxides, azo compounds) | Coordination of monomers to a metal catalyst (e.g., Ziegler-Natta, metallocene catalysts) |
| Monomer Types | Primarily vinyl monomers (e.g., styrene, acrylates) | Alpha-olefins (e.g., ethylene, propylene) |
| Polymer Structure | Random branching, broad molecular weight distribution | Highly stereoregular, controlled molecular weight and architecture |
| Control over Polymerization | Limited control over tacticity; broad polydispersity index | Precise control over stereochemistry and tacticity |
| Reaction Conditions | Often requires high temperatures and radical initiators | Usually conducted under milder conditions with catalyst |
| Applications | General-purpose plastics, adhesives, coatings | High-performance polymers, elastomers, fibers |
| Advantages | Simple, versatile, tolerant to impurities | High specificity, stereoregularity, and polymer property control |
| Disadvantages | Less control over polymer microstructure and properties | Requires expensive catalysts and rigorous conditions |
Introduction to Polymerization Methods
Free radical polymerization is a widely used method involving the initiation of polymer chains through free radicals, typically generating polymers with broad molecular weight distributions and random monomer sequencing. Coordination polymerization employs transition metal catalysts, enabling precise control over polymer architecture, stereochemistry, and molecular weight, which results in polymers with uniform properties and crystallinity. These distinct polymerization methods cater to diverse industrial applications by tuning polymer characteristics from elasticity to thermal resistance.
Overview of Free Radical Polymerization
Free radical polymerization is a widely used method for creating polymers by initiating the polymer chain growth through free radicals generated from initiators like benzoyl peroxide or AIBN. This process proceeds via three key steps: initiation, propagation, and termination, allowing for the polymerization of vinyl monomers such as styrene, methyl methacrylate, and acrylates. Despite its versatility and simplicity, free radical polymerization often results in polymers with broad molecular weight distributions and limited control over stereochemistry compared to coordination polymerization.
Overview of Coordination Polymerization
Coordination polymerization is a catalytic process primarily used to synthesize polyolefins such as polyethylene and polypropylene with precise control over molecular weight and stereochemistry. It involves transition metal catalysts like Ziegler-Natta or metallocenes that coordinate to the monomer, facilitating insertion polymerization through metal-carbon bonds. This method produces polymers with narrow molecular weight distribution and highly regular chain structures, unlike the broad and random polymers formed by free radical polymerization.
Mechanisms: Free Radical vs Coordination Polymerization
Free radical polymerization involves the initiation step by free radicals generated from initiators such as peroxides, which propagate chain growth through successive addition to monomer double bonds. Coordination polymerization uses transition metal catalysts, like Ziegler-Natta or metallocene catalysts, where the monomer coordinates to the metal center before insertion into the growing polymer chain, allowing precise control over stereochemistry and molecular weight. The key mechanistic difference lies in the radical-driven random addition in free radical polymerization versus the metal-centered, controlled insertion process in coordination polymerization.
Types of Monomers Used in Each Method
Free radical polymerization primarily uses vinyl monomers such as styrene, acrylates, and vinyl chloride, which contain carbon-carbon double bonds susceptible to radical initiation. Coordination polymerization is suited for a-olefins like ethylene and propylene, utilizing transition metal catalysts to control polymer structure and stereochemistry. Understanding the monomer types appropriate for each method helps you select optimal polymerization processes for desired material properties.
Polymer Structure and Properties Comparison
Free radical polymerization typically results in polymers with random chain branching and broad molecular weight distributions, producing materials with lower crystallinity and mechanical strength. Coordination polymerization, catalyzed by transition metals, generates polymers with highly regular stereoregularity, narrow molecular weight distribution, and enhanced crystallinity, leading to superior tensile strength and thermal stability. These structural differences fundamentally influence polymer properties, making coordination polymers ideal for high-performance applications requiring precise control over polymer architecture.
Advantages and Limitations of Free Radical Polymerization
Free radical polymerization offers the advantage of versatility, allowing polymerization of a wide range of monomers under relatively mild conditions and at low cost, making it industrially significant for producing polymers like polyethylene and polystyrene. Limitations include poor control over molecular weight distribution, resulting in polymers with broad polydispersity and limited stereoregularity, which affects material properties. The process is also sensitive to inhibitors and chain transfer reactions, leading to termination and reduced polymerization efficiency.
Advantages and Limitations of Coordination Polymerization
Coordination polymerization offers high stereochemical control and the ability to produce polymers with precise molecular weight distribution, making it ideal for manufacturing highly regular polymers such as isotactic polypropylene. Its advantages include lower reaction temperatures and the production of polymers with superior mechanical and thermal properties compared to free radical polymerization. However, limitations include the sensitivity of catalysts to impurities and moisture, higher catalyst costs, and restrictions to specific monomers, which can limit versatility and increase processing complexity.
Industrial Applications and Practical Uses
Free radical polymerization is widely used in the production of polymers such as polystyrene, polyvinyl chloride (PVC), and polymethyl methacrylate (PMMA), making it ideal for applications in packaging, coatings, and adhesives due to its versatility and cost-effectiveness. Coordination polymerization, leveraging catalysts like Ziegler-Natta and metallocenes, excels in manufacturing highly crystalline polymers like linear polyethylene and isotactic polypropylene, crucial for automotive parts, containers, and fibers with enhanced mechanical properties. Your choice between these methods depends on the desired polymer structure and performance requirements in specific industrial applications.
Future Trends in Polymerization Techniques
Future trends in polymerization techniques emphasize the development of more sustainable and precise methods, with coordination polymerization advancing through novel catalysts that enable better control over polymer architecture and stereochemistry. Free radical polymerization is evolving with enhanced initiators and controlled radical polymerization strategies to improve polymer uniformity and functionality. Integrating machine learning and green chemistry principles is driving innovation, aiming for more efficient, eco-friendly, and application-specific polymer production.
Free radical polymerization vs coordination polymerization Infographic
 libmatt.com
libmatt.com