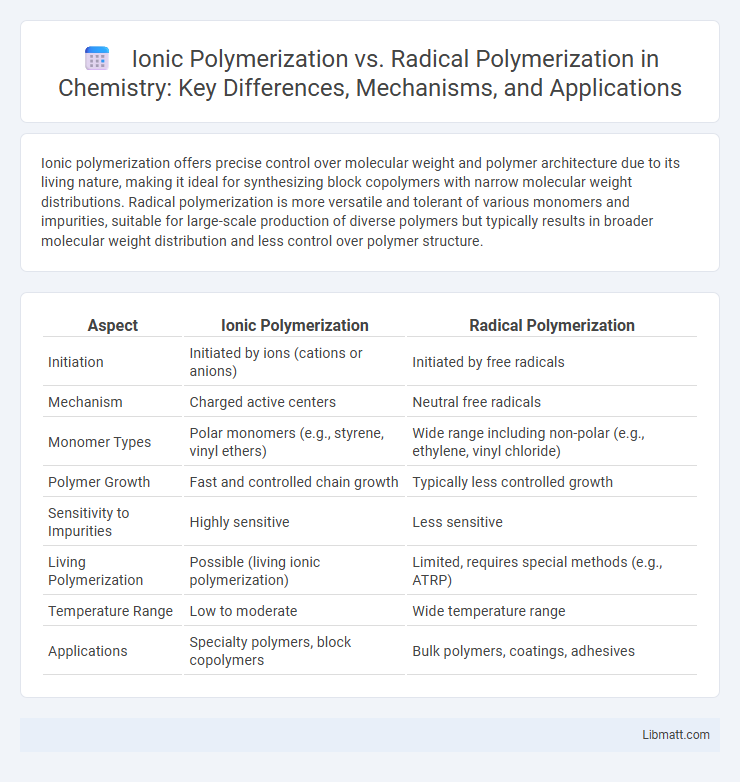
Ionic polymerization offers precise control over molecular weight and polymer architecture due to its living nature, making it ideal for synthesizing block copolymers with narrow molecular weight distributions. Radical polymerization is more versatile and tolerant of various monomers and impurities, suitable for large-scale production of diverse polymers but typically results in broader molecular weight distribution and less control over polymer structure.
Table of Comparison
| Aspect | Ionic Polymerization | Radical Polymerization |
|---|---|---|
| Initiation | Initiated by ions (cations or anions) | Initiated by free radicals |
| Mechanism | Charged active centers | Neutral free radicals |
| Monomer Types | Polar monomers (e.g., styrene, vinyl ethers) | Wide range including non-polar (e.g., ethylene, vinyl chloride) |
| Polymer Growth | Fast and controlled chain growth | Typically less controlled growth |
| Sensitivity to Impurities | Highly sensitive | Less sensitive |
| Living Polymerization | Possible (living ionic polymerization) | Limited, requires special methods (e.g., ATRP) |
| Temperature Range | Low to moderate | Wide temperature range |
| Applications | Specialty polymers, block copolymers | Bulk polymers, coatings, adhesives |
Introduction to Polymerization Mechanisms
Ionic polymerization involves the initiation and propagation of polymer chains through charged intermediates, either cations or anions, enabling precise control over polymer architecture and molecular weight. Radical polymerization proceeds via free radical intermediates generated through initiators, leading to rapid chain growth but with less control over polymer structure and molecular weight distribution. Understanding these distinct mechanisms allows you to select the optimal polymerization process based on desired polymer properties and application requirements.
Overview of Ionic Polymerization
Ionic polymerization involves the growth of polymer chains through ionic active centers, either cations in cationic polymerization or anions in anionic polymerization, allowing for precise control over molecular weight and polymer architecture. This process is highly sensitive to impurities and requires stringent reaction conditions, yet it enables the synthesis of polymers with narrow molecular weight distributions and complex structures. Your choice of ionic polymerization can lead to advanced materials with tailored properties, distinct from those produced by the more tolerant but less controlled radical polymerization technique.
Fundamentals of Radical Polymerization
Radical polymerization involves the initiation, propagation, and termination of free radical species generated typically by thermal decomposition of initiators like benzoyl peroxide or AIBN. This mechanism is characterized by the formation of highly reactive chain carriers that add monomers with unsaturated bonds, predominantly alkenes, through a chain-growth process. The radical nature allows for rapid polymerization rates but results in broad molecular weight distributions due to irreversible termination steps such as combination or disproportionation.
Key Differences Between Ionic and Radical Polymerization
Ionic polymerization involves the initiation by ions, leading to highly controlled polymer chain growth with narrow molecular weight distribution, while radical polymerization uses free radicals as initiators, resulting in broader molecular weight distribution and less control over chain length. Ionic polymerization allows for living polymerization techniques with precise molecular architecture, whereas radical polymerization typically involves termination reactions that limit control over polymer structure. The sensitivity of ionic polymerization to impurities contrasts with the tolerance of radical polymerization to a wider range of reaction conditions and monomer types.
Types of Ionic Polymerization: Cationic vs. Anionic
Cationic polymerization involves positively charged ions as active centers, typically using monomers like isobutylene or styrene with Lewis acids as initiators, enabling rapid polymerization and high molecular weight polymers. Anionic polymerization, driven by negatively charged ions, utilizes strong bases such as organolithium compounds to initiate polymerization of monomers like butadiene or styrene, offering precise control over molecular weight and polymer architecture. Understanding the distinctions between cationic and anionic mechanisms allows you to select the optimal polymerization route for tailored polymer properties and applications.
Initiators in Ionic and Radical Polymerization
Initiators in ionic polymerization typically involve strong nucleophiles or electrophiles such as organometallic compounds or Lewis acids, which generate charged active centers for chain growth. Radical polymerization initiators commonly include organic peroxides or azo compounds that decompose to form free radicals, initiating polymer chain formation. The choice of initiator critically influences polymer structure, molecular weight, and reaction conditions in both polymerization methods.
Polymerization Conditions and Control
Ionic polymerization operates under stringent moisture-free and low-temperature conditions to maintain high control over molecular weight and polymer architecture, whereas radical polymerization is more tolerant of environmental variables but offers less precision in polymer structure. Ionic methods, such as anionic or cationic polymerization, require careful handling of initiators and solvents to avoid premature termination, enabling the synthesis of polymers with narrow molecular weight distributions. Your choice between these techniques depends on the desired polymer properties and tolerance for reaction conditions, with ionic polymerization excelling in controlled polymer growth and radical polymerization being more versatile and easier to implement.
Advantages and Limitations of Each Method
Ionic polymerization offers advantages such as precise control over molecular weight and polymer architecture due to its living polymerization nature, enabling the synthesis of block copolymers with narrow molecular weight distribution; however, it requires stringent anhydrous and oxygen-free conditions, limiting its practicality for large-scale industrial applications. Radical polymerization is more versatile and tolerant of impurities, making it suitable for a wide range of monomers and industrial processes, yet it often results in broader molecular weight distribution and less control over polymer structure due to chain termination and transfer reactions. The choice between ionic and radical polymerization depends on the desired polymer properties, reaction conditions, and scalability requirements.
Applications in Industry and Research
Ionic polymerization enables precise control over molecular weight and polymer architecture, making it ideal for producing specialty elastomers, adhesives, and advanced plastics in industries such as automotive and aerospace. Radical polymerization dominates large-scale production of polymers like polyethylene, polystyrene, and polyvinyl chloride due to its versatility and cost-effectiveness, widely used in packaging, construction, and consumer goods. Research often explores ionic polymerization for synthesizing block copolymers and complex polymer networks, while radical polymerization remains central to developing new monomers and copolymer systems for innovative material solutions.
Conclusion: Selecting the Right Polymerization Technique
Choosing between ionic polymerization and radical polymerization depends on the desired polymer structure and properties; ionic polymerization offers precise control over molecular weight and polymer architecture, producing polymers with narrow molecular weight distribution, while radical polymerization is versatile and tolerant of a wide range of monomers and impurities but results in broader molecular weight distribution. Your application requirements, such as polymer uniformity, reaction conditions, and monomer sensitivity, will guide the selection of the optimal polymerization technique. Understanding these differences ensures efficient synthesis of polymers tailored to specific functional and performance needs.
ionic polymerization vs radical polymerization Infographic
 libmatt.com
libmatt.com